Search
- Page Path
- HOME > Search
- [Korean]
- Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
- Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2018;25(5):420-425. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.420

- 923 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Core-shell structured nanoparticles are garnering attention because these nanoparticles are expected to have a wide range of applications. The objective of the present study is to improve the coating efficiency of gold shell formed on the surface of silica nanoparticles for SiO2@Au core-shell structure. For the efficient coating of gold shell, we attempt an in-situ synthesis method such that the nuclei of the gold nanoparticles are generated and grown on the surface of silica nanoparticles. This method can effectively form a gold shell as compared to the conventional method of attaching gold nanoparticles to silica particles. It is considered possible to form a dense gold shell because the problems caused by electrostatic repulsion between the gold nanoparticles in the conventional method are eliminated.
- [Korean]
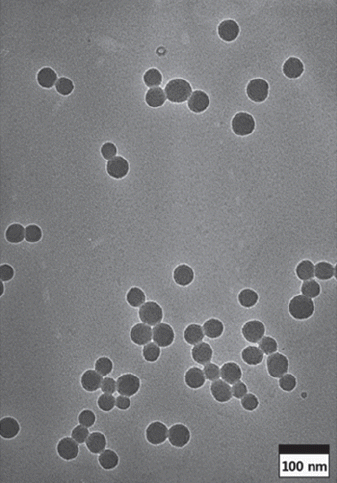
- Effect of Reaction Parameters on Silica Nanoparticles Synthesized by Sol-gel Method
- Young-Hyun Lim, Do Kyung Kim, Young-Keun Jeong
- J Korean Powder Metall Inst. 2016;23(6):442-446. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.442
- 3,027 View
- 47 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The sol-gel method is the simplest method for synthesizing monodispersed silica particles. The purpose of this study is to synthesize uniform, monodisperse spherical silica nanoparticles using tetraethylorthosilicate (TEOS) as the silica precursor, ethanol, and deionized water in the presence of ammonia as a catalyst. The reaction time and temperature and the concentration of the reactants are controlled to investigate the effect of the reaction parameters on the size of the synthesized particles. The size and morphology of the obtained silica particles are investigated using transmission electron microscopy and particle size analysis. The results show that monodispersed silica particles over a size range of 54-504 nm are successfully synthesized by the sol-gel method without using any additional process. The nanosized silica particles can be synthesized at higher TEOS/H2O ratios, lower ammonia concentrations, and especially, higher reaction temperatures.
-
Citations
Citations to this article as recorded by- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
Mustapha Sulaiman, Naseer Inuwa Durumin Iya, Mamudu Aliyu
FUDMA JOURNAL OF SCIENCES.2024; 8(3): 222. CrossRef - Nanostructure Construction of SiO2@Au Core-Shell by In-situ Synthesis
Mu-Jae Pyeon, Do Kyung Kim, Young-Keun Jeong
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 420. CrossRef
- SYNTHESIS OF SILICA NANOPARTICLES FROM SUGARCANE WASTE: PRECIPITATION-BASED SIZE CONTROL AND CHARACTERIZATION
TOP
 KPMI
KPMI


 First
First Prev
Prev


