Search
- Page Path
- HOME > Search
- [Korean]
- A Study on Rinsing Effects of Sn Sensitization and Pd Activation Processes for Uniform Electroless Plating
- Seong-Jae Jeong, Mi-Se Chang, Jae-Won Jeong, Sang-Sun Yang, Young-Tae Kwon
- J Powder Mater. 2022;29(6):511-516. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.511
- 1,750 View
- 14 Download
-
 Abstract
Abstract
 PDF
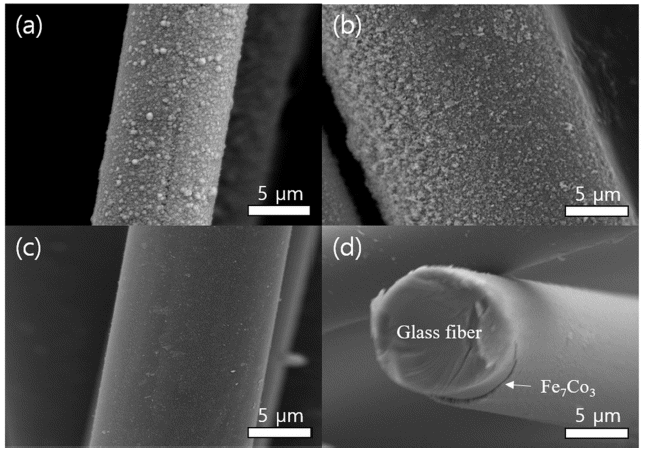
PDF Electroless plating is widely utilized in engineering for the metallization of insulator substrates, including polymers, glass, and ceramics, without the need for the application of external potential. Homogeneous nucleation of metals requires the presence of Sn-Pd catalysts, which significantly reduce the activation energy of deposition. Therefore, rinsing conducted during Sn sensitization and Pd activation is a key variable for the formation of a uniform seed layer without the lack or excess of catalysts. Herein, we report the optimized rinsing process for the functionalization of Sn-Pd catalysts, which enables the uniform FeCo metallization of the glass fibers. Rinsing enables good deposition of the FeCo alloy because of the removal of excess catalysts from the glass fiber. Concurrently, excessive rinsing results in a complete removal of the Sn–Pd nucleus. Collectively, the comprehensive study of the proposed nanomaterial preparation and surface science show that the metallization of insulators is a promising technology for electronics, solar cells, catalysts, and mechanical parts.
- [Korean]
- Thermophysical Properties of Copper/graphite Flake Composites by Electroless Plating and Spark Plasma Sintering
- Jaesung Lee, Ji Yeon Kang, Seulgi Kim, Chanhoe Jung, Dongju Lee
- J Korean Powder Metall Inst. 2020;27(1):25-30. Published online February 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.1.25
- 1,355 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
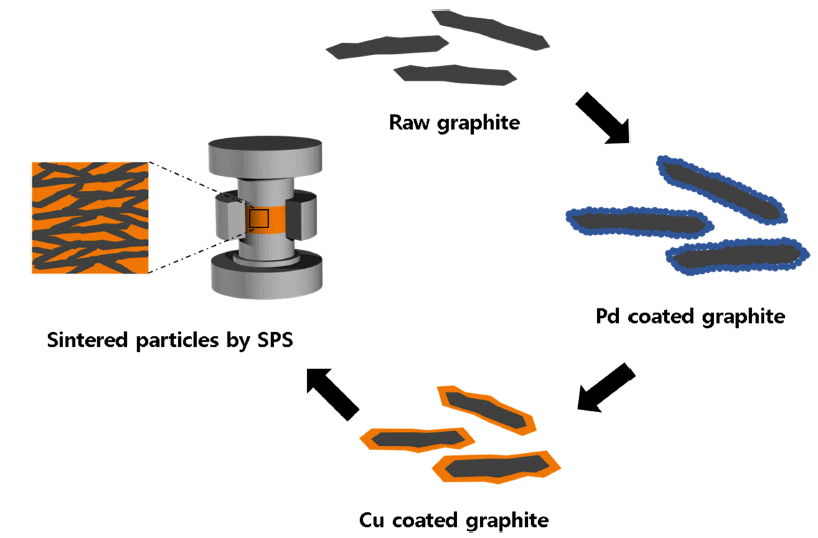
PDF Recently, the amount of heat generated in devices has been increasing due to the miniaturization and high performance of electronic devices. Cu-graphite composites are emerging as a heat sink material, but its capability is limited due to the weak interface bonding between the two materials. To overcome these problems, Cu nanoparticles were deposited on a graphite flake surface by electroless plating to increase the interfacial bonds between Cu and graphite, and then composite materials were consolidated by spark plasma sintering. The Cu content was varied from 20 wt.% to 60 wt.% to investigate the effect of the graphite fraction and microstructure on thermal conductivity of the Cu-graphite composites. The highest thermal conductivity of 692 W m−1K−1 was achieved for the composite with 40 wt.% Cu. The measured coefficients of thermal expansion of the composites ranged from 5.36 × 10−6 to 3.06 × 10−6 K−1. We anticipate that the Cu-graphite composites have remarkable potential for heat dissipation applications in energy storage and electronics owing to their high thermal conductivity and low thermal expansion coefficient.
-
Citations
Citations to this article as recorded by- Experimental and numerical investigation of thermally stable Cu/Mo/Cu spacer with low-CTE and high-k for double side cooling power semiconductor modules
YehRi Kim, Byeongchan Kim, Kue Jin Han, Taeseong Han, Dongjin Kim
Applied Thermal Engineering.2026; 288: 129661. CrossRef
- Experimental and numerical investigation of thermally stable Cu/Mo/Cu spacer with low-CTE and high-k for double side cooling power semiconductor modules
- [Korean]
- Synthesis of Nickel Nanoparticle-adsorbed Aluminum Powders for Energetic Applications
- Dong Won Kim, Gu Hyun Kwon, Kyung Tae Kim
- J Korean Powder Metall Inst. 2016;24(3):242-247. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2017.24.3.242

- 1,239 View
- 8 Download
- 8 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, the electroless nickel plating method has been investigated for the coating of Ni nanoparticles onto fine Al powder as promising energetic materials. The adsorption of nickel nanoparticles onto the surface of Al powders has been studied by varying various process parameters, namely, the amounts of reducing agent, complexing agent, and pH-controller. The size of nickel nanoparticles synthesized in the process has been optimized to approximately 200 nm and they have been adsorbed on the Al powder. TGA results clearly show that the temperature at which oxidation of Al mainly occurs is lowered as the amount of Ni nanoparticles on the Al surface increases. Furthermore, the Ni-plated Al powders prepared for all conditions show improved exothermic reaction due to the selfpropagating high-temperature synthesis (SHS) between Ni and Al. Therefore, Al powders fully coated by Ni nanoparticles show the highest exothermic reactivity: this demonstrates the efficiency of Ni coating in improving the energetic properties of Al powders.
-
Citations
Citations to this article as recorded by- Synthesis and oxidation behavior of Al@Ni-Fe core-shell energetic composite powders
Khawar Yaqoob, Asifa Kusar, Amena Mohsin, Onur Ertuğrul, Ahmet Çağrı Kılınç, Fahad Ali, Rub Nawaz Shahid, Naeem ul Haq Tariq, Hassan Wahab, Hasan Bin Awais
Energetic Materials Frontiers.2025;[Epub] CrossRef - Study Progress in Improving the Energy Release Rate of Al Powders
永鹏 陈
Material Sciences.2024; 14(08): 1178. CrossRef - Study on the Combustion Characteristics of Ethanol Nanofuel
Kwanyoung Noh, Hyemin Kim, Siwook Nam, Soonho Song
Aerospace.2023; 10(10): 878. CrossRef - The Self-Reduction during the Thermal Decomposition of an Ammonium Molybdate
Kyoungkeun Yoo, Won Beom Koo, Hanggoo Kim, Sang-hun Lee
Minerals.2023; 13(2): 133. CrossRef - Increased exothermic reactivity of polytetrafluoroethylene-coated aluminum powders: Impact of powder size reduction
Soo-ho Jung, Kyung Tae Kim, Jinhee Bae, Yoon Jeong Choi, Jae Min Kim, Jeong-Yun Sun
Materials Letters.2023; 351: 135009. CrossRef - High energy Al@Ni preparation of core-shell particles by adjusting nickel layer thickness
Yongpeng Chen, Jianguo Zhang, Jiawei Zhu, Ning Xiang, Huichao Zhang, Zunning Zhou
Vacuum.2022; 205: 111344. CrossRef - Electroless deposition of Ni nanoparticles on micron-sized boron carbide particles: Physicochemical and oxidation properties
Prashant Ravasaheb Deshmukh, Hyung Soo Hyun, Youngku Sohn, Weon Gyu Shin
Korean Journal of Chemical Engineering.2020; 37(3): 546. CrossRef - Synthesis and exothermic reactions of ultra-fine snowman-shaped particles with directly bonded Ni/Al interfaces
Gu Hyun Kwon, Kyung Tae Kim, Dong Won Kim, Jungho Choe, Jung Yeul Yun, Jong-Man Kim
Applied Surface Science.2019; 476: 481. CrossRef
- Synthesis and oxidation behavior of Al@Ni-Fe core-shell energetic composite powders
- [Korean]
- Thermal Properties of Diamond Aligned Electroless Ni Plating Layer/Oxygen Free Cu Substrates
- Da-Woon Jeong, Song-Yi Kim, Kyoung-Tae Park, Seok-Jun Seo, Taek Soo Kim, Bum Sung Kim
- J Korean Powder Metall Inst. 2015;22(2):134-137. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.134
- 1,020 View
- 4 Download
-
 Abstract
Abstract
 PDF
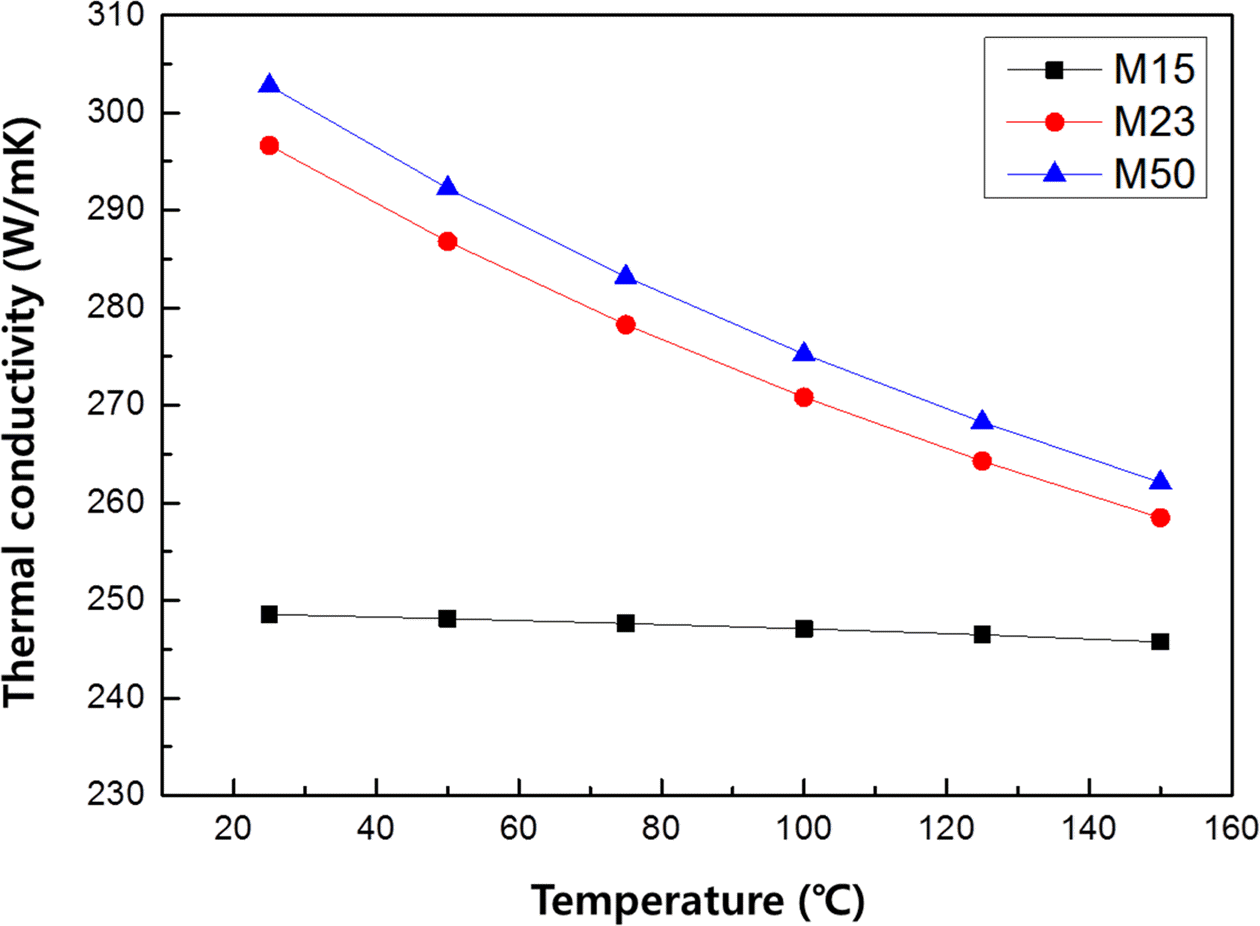
PDF The monolayer engineering diamond particles are aligned on the oxygen free Cu plates with electroless Ni plating layer. The mean diamond particle sizes of 15, 23 and 50 μm are used as thermal conductivity pathway for fabricating metal/carbon multi-layer composite material systems. Interconnected void structure of irregular shaped diamond particles allow dense electroless Ni plating layer on Cu plate and fixing them with 37-43% Ni thickness of their mean diameter. The thermal conductivity decrease with increasing measurement temperature up to 150°C in all diamond size conditions. When the diamond particle size is increased from 15 μm to 50 μm (Max. 304 W/mK at room temperature) tended to increase thermal conductivity, because the volume fraction of diamond is increased inside plating layer.
TOP
 KPMI
KPMI


 First
First Prev
Prev


