Search
- Page Path
- HOME > Search
- [Korean]
- Magnetic Properties of Micron Sized Fe3O4 Crystals Synthesized by Hydrothermal Methods
- Ki-Bum Lee, Chunghee Nam
- J Korean Powder Metall Inst. 2019;26(6):481-486. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.481
- 1,161 View
- 9 Download
-
 Abstract
Abstract
 PDF
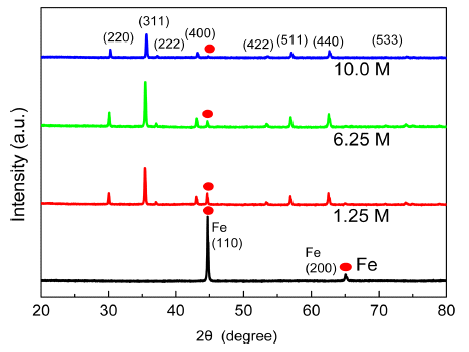
PDF Iron oxides currently attract considerable attention due to their potential applications in the fields of lithiumion batteries, bio-medical sensors, and hyperthermia therapy materials. Magnetite (Fe3O4) is a particularly interesting research target due to its low cost, good biocompatibility, outstanding stability in physiological conditions. Hydrothermal synthesis is one of several liquid-phase synthesis methods with water or an aqueous solution under high pressure and high temperature. This paper reports the growth of magnetic Fe3O4 particles from iron powder (spherical, <10 μm) through an alkaline hydrothermal process under the following conditions: (1) Different KOH molar concentrations and (2) different synthesis time for each KOH molar concentrations. The optimal condition for the synthesis of Fe3O4 using Fe powders is hydrothermal oxidation with 6.25 M KOH for 48 h, resulting in 89.2 emu/g of saturation magnetization at room temperature. The structure and morphologies of the synthesized particles are characterized by X-ray diffraction (XRD, 2θ = 20°–80°) with Cu-kα radiation and field emission scanning electron microscopy (FE-SEM), respectively. The magnetic properties of magnetite samples are investigated using a vibrating sample magnetometer (VSM). The role of KOH in the formation of magnetite octahedron is observed.
- [English]
- The Synthesis and Photocatalytic activity of Carbon Nanotube-mixed TiO2 Nanotubes
- Chun Woong Park, Young Do Kim, Tohru Sekino, Se Hoon Kim
- J Korean Powder Metall Inst. 2017;24(4):279-284. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.279
- 1,543 View
- 11 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
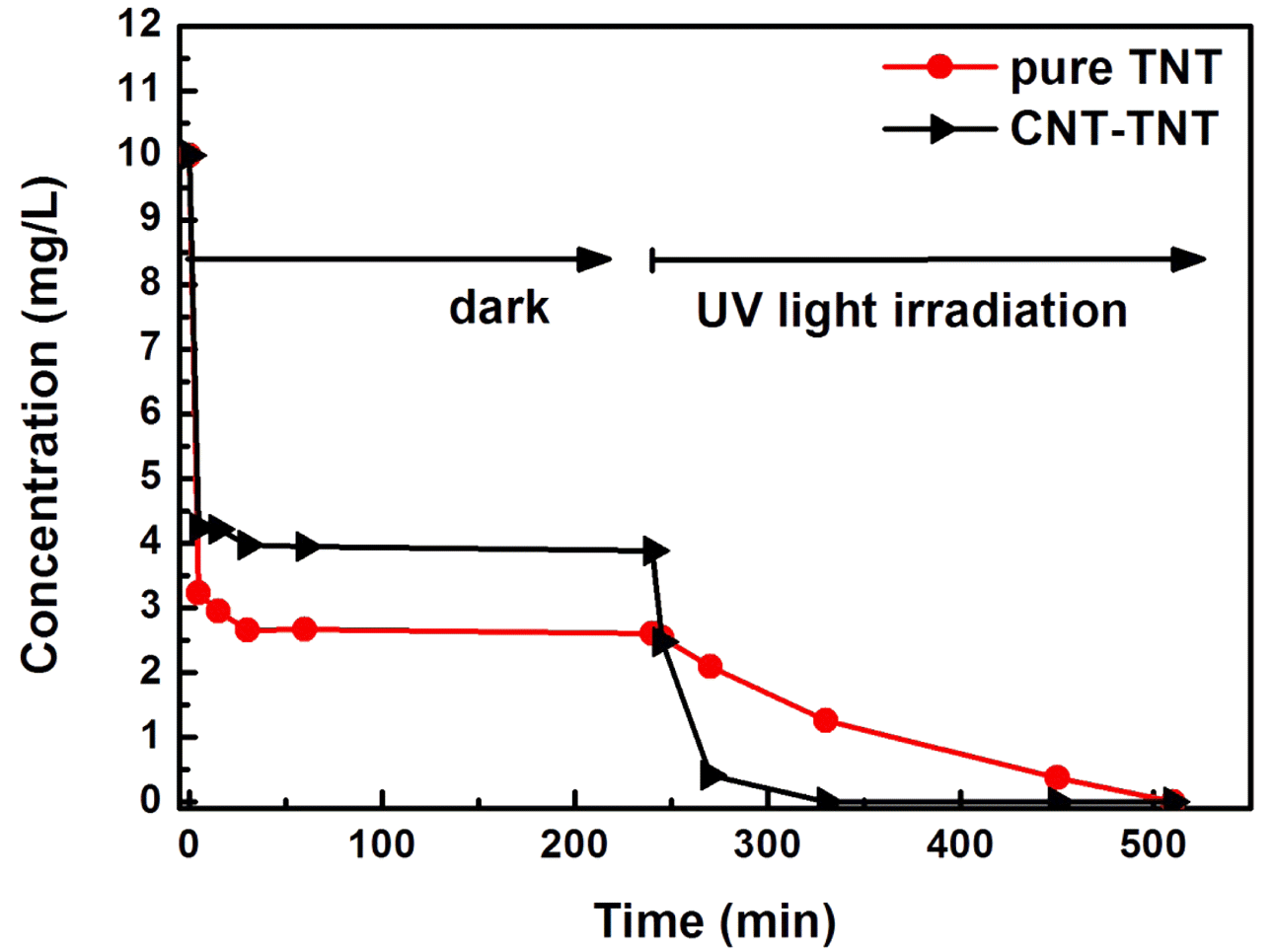
PDF The formation mechanism and photocatalytic properties of a multiwalled carbon nanotube (MWCNT)/TiO2- based nanotube (TNTs) composite are investigated. The CNT/TNT composite is synthesized via a solution chemical route. It is confirmed that this 1-D nanotube composite has a core-shell nanotubular structure, where the TNT surrounds the CNT core. The photocatalytic activity investigated based on the methylene blue degradation test is superior to that of with pure TNT. The CNTs play two important roles in enhancing the photocatalytic activity. One is to act as a template to form the core-shell structure while titanate nanosheets are converted into nanotubes. The other is to act as an electron reservoir that facilitates charge separation and electron transfer from the TNT, thus decreasing the electronhole recombination efficiency.
-
Citations
Citations to this article as recorded by- Carbon-based photocatalysis in organic transformation
Md Razu Ahmed, Yuta Nishina
Bulletin of the Chemical Society of Japan.2026;[Epub] CrossRef - Low-Dimensional Carbon and Titania Nanotube Composites via a Solution Chemical Process and Their Nanostructural and Electrical Properties for Electrochemical Devices
Sunghun Eom, Sung Hun Cho, Tomoyo Goto, Myoung Pyo Chun, Tohru Sekino
ACS Applied Nano Materials.2019; 2(10): 6230. CrossRef
- Carbon-based photocatalysis in organic transformation
- [Korean]
- Synthesis of Perforated Polygonal Cobalt Oxides using a Carbon Nanofiber Template
- Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2015;22(5):350-355. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.350
- 831 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
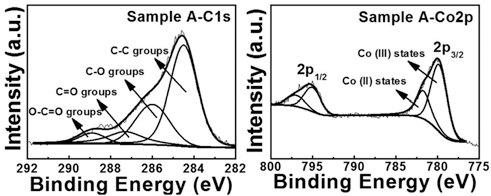
PDF Perforated polygonal cobalt oxide (CO3O4) is synthesized using electrospinning and a hydrothermal method followed by the removal of a carbon nanofiber (CNF) template. To investigate their formation mechanism, thermogravimetric analysis, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and Xray photoelectron spectroscopy are examined. To obtain the optimum condition of perforated polygonal CO3O4, we prepare three different weight ratios of the Co precursor and the CNF template: sample A (Co precursor:CNF template- 10:1), sample B (Co precursor:CNF template-3.2:1), and sample C (Co precursor:CNF template-2:1). Among them, sample A exhibits the perforated polygonal CO3O4 with a thin carbon layer (5.7-6.2 nm) owing to the removal of CNF template. However, sample B and sample C synthesized perforated round CO3O4 and destroyed CO3O4 powders, respectively, due to a decreased amount of Co precursor. The increased amount of the CNF template prevents the formation of polygonal CO3O4. For sample A, the optimized weight ratio of the Co precursor and CNF template may be related to the successful formation of perforated polygonal CO3O4. Thus, perforated polygonal CO3O4 can be applied to electrode materials of energy storage devices such as lithium ion batteries, supercapacitors, and fuel cells.
-
Citations
Citations to this article as recorded by- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef - Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef
- Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
TOP
 KPMI
KPMI


 First
First Prev
Prev


