Search
- Page Path
- HOME > Search
- [English]
- Bandgap Tuning and Quenching Effects of In(Zn)P@ZnSe@ZnS Quantum Dots
- Sang Yeon Lee, Su Hyun Park, Gyungsu Byun, Chang-Yeoul Kim
- J Powder Mater. 2024;31(3):226-235. Published online June 27, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00003

- 3,016 View
- 44 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - InP quantum dot (QDs) have attracted researchers’ interest due to their applicability in quantum dot light-emitting displays (QLED) or biomarkers for detecting cancers or viruses. The surface or interface control of InP QD core/shell has substantially increased quantum efficiency, with a quantum yield of 100% reached by introducing HF to inhibit oxide generation. In this study, we focused on the control of bandgap energy of quantum dots by changing the Zn/(In+Zn) ratio in the In(Zn)P core. Zinc incorporation can change the photoluminescent light colors of green, yellow, orange, and red. Diluting a solution of as-synthesized QDs by more than 100 times did not show any quenching effects by the Förster resonance energy transfer phenomenon between neighboring QDs.
-
Citations
Citations to this article as recorded by- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
Hongcheng Yang, Junjie Hao, Mingyu Sun, Yujie Song, Kai Wang, Yujie Song, Xiao Wei Sun, Wenda Zhang
The Innovation.2026; 7(2): 101121. CrossRef
- Enhancing luminescence of QD thin films, polymer composite films, and LED devices by nanostructures
- [Korean]
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(6):489-493. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.489
- 1,596 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
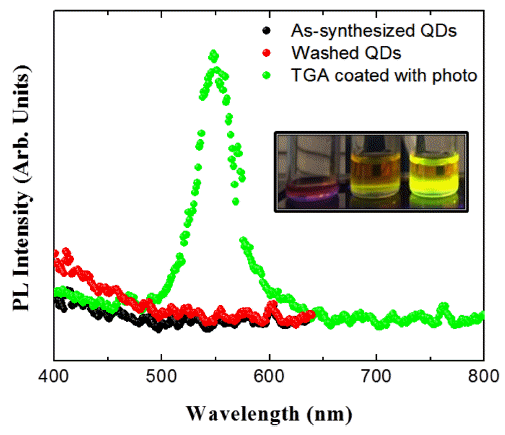
PDF In this study, the surface passivation process for InP-based quantum dots (QDs) is investigated. Surface coating is performed with poly(methylmethacrylate) (PMMA) and thioglycolic acid. The quantum yield (QY) of a PMMA-coated sample slightly increases by approximately 1.3% relative to that of the as-synthesized InP/ZnS QDs. The QYs of the uncoated and PMMA-coated samples drastically decrease after 16 days because of the high defect state density of the InP-based QDs. PMMA does not have a significant effect on the defect passivation. Thioglycolic acid is investigated in this study for the effective surface passivation of InP-based QDs. Surface passivation with thioglycolic acid is more effective than that with the PMMA coating, and the QY increases from 1.7% to 11.3%. ZnS formed on the surface of the InP QDs and S in thioglycolic acid show strong bonding property. Additionally, the QY is further increased up to 21.0% by the photochemical reaction. Electron–hole pairs are formed by light irradiation and lead to strong bonding between the inorganic and thioglycolic acid sulfur. The surface of the InP core QDs, which does not emit light, is passivated by the irradiated light and emits green light after the photochemical reaction.
-
Citations
Citations to this article as recorded by- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef
- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
- [Korean]
- Luminescence Properties of InP/ZnS Quantum Dots depending on InP Core synthesis Temperature
- Han Wook Seo, Da-Woon Jeong, Min Young Kim, Seoung Kyun Hyun, Ji Sun On, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(4):321-325. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.321
- 1,469 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
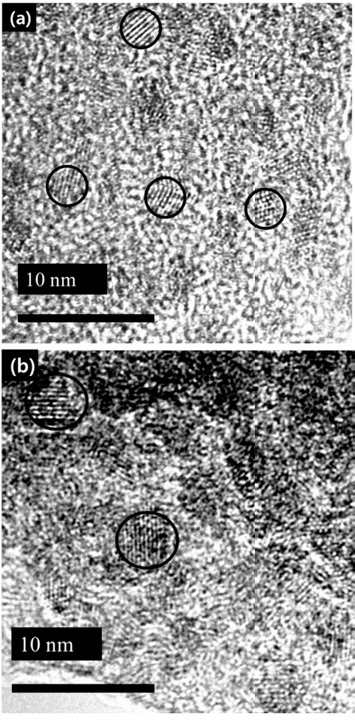
PDF In this study, we investigate the optical properties of InP/ZnS core/shell quantum dots (QDs) by controlling the synthesis temperature of InP. The size of InP determined by the empirical formula tends to increase with temperature: the size of InP synthesized at 140oC and 220oC is 2.46 nm and 4.52 nm, respectively. However, the photoluminescence (PL) spectrum of InP is not observed because of the formation of defects on the InP surface. The growth of InP is observed during the deposition of the shell (ZnS) on the synthesized InP, which is ended up with green-red PL spectrum. We can adjust the PL spectrum and absorption spectrum of InP/ZnS by simply adjusting the core temperature. Thus, we conclude that there exists an optimum shell thickness for the QDs according to the size.
-
Citations
Citations to this article as recorded by- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- [Korean]
- Growth mechanism of InP and InP/ZnS synthesis using colloidal synthesis
- Han wook Seo, Da-woon Jeong, Bin Lee, Seoung kyun Hyun, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(1):6-10. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.6
- 1,448 View
- 5 Download
-
 Abstract
Abstract
 PDF
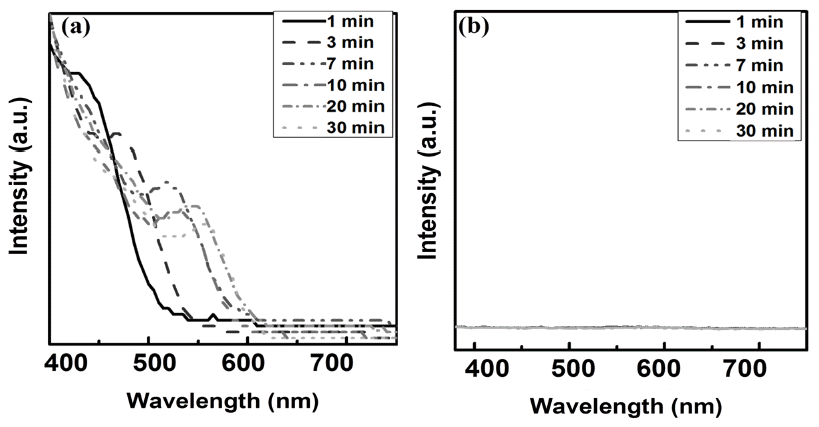
PDF This study investigates the main growth mechanism of InP during InP/ZnS reaction of quantum dots (QDs). The size of the InP core, considering a synthesis time of 1-30 min, increased from the initial 2.56 nm to 3.97 nm. As a result of applying the proposed particle growth model, the migration mechanism, with time index 7, was found to be the main reaction. In addition, after the removal of unreacted In and P precursors from bath, further InP growth (of up to 4.19 nm (5%)), was observed when ZnS was added. The full width at half maximum (FWHM) of the synthesized InP/ZnS quantum dots was found to be relatively uniform, measuring about 59 nm. However, kinetic growth mechanism provides limited information for InP / ZnS core shell QDs, because the surface state of InP changes with reaction time. Further study is necessary, in order to clearly determine the kinetic growth mechanism of InP / ZnS core shell QDs.
- [Korean]
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,029 View
- 8 Download
-
 Abstract
Abstract
 PDF
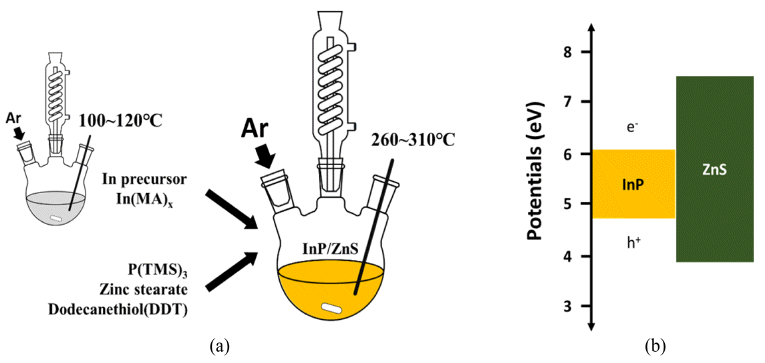
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
- Improved Luminescent Characterization and Synthesis of InP/ZnS Quantum Dot with High-Stability Precursor
- Eun-Jin Lee, Jong-Woo Moon, Yang-Do Kim, Pyung-Woo Shin, Young-Kuk Kim
- J Korean Powder Metall Inst. 2015;22(6):385-390. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.385
- 1,114 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF We report a synthesis of non-toxic InP nanocrystals using non-pyrolytic precursors instead of pyrolytic and unstable tris(trimethylsilyl)phosphine, a popular precursor for synthesis of InP nanocrystals. In this study, InP nanocrystals are successfully synthesized using hexaethyl phosphorous triamide (HPT) and the synthesized InP nanocrystals showed a broad and weak photoluminescence (PL) spectrum. As synthesized InP nanocrystals are subjected to further surface modification process to enhance their stability and photoluminescence. Surface modification of InP nanocrystals is done at 230°C using 1-dodecanethiol, zinc acetate and fatty acid as sources of ZnS shell. After surface modification, the synthesized InP/ZnS nanocrystals show intense PL spectra centered at the emission wavelength 612 nm through 633 nm. The synthesized InP/ZnS core/shell structure is confirmed with X-ray diffraction (XRD) and Inductively Coupled Plasma - Atomic Emission Spectrometer (ICP-AES). After surface modification, InP/ZnS nanocrystals having narrow particle size distribution are observed by Field Emission Transmission Electron Microscope (FE-TEM). In contrast to uncapped InP nanocrystals, InP/ZnS nanocrystals treated with a newly developed surface modified procedure show highly enhanced PL spectra with quantum yield of 47%.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef - Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(1): 11. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
TOP
 KPMI
KPMI


 First
First Prev
Prev


