Search
- Page Path
- HOME > Search
- [English]
- A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
- Jei-Pil Wang, Gyu-Cheol Kim, Min-Seok Go
- J Korean Powder Metall Inst. 2020;27(4):287-292. Published online August 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.4.287
- 1,814 View
- 23 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
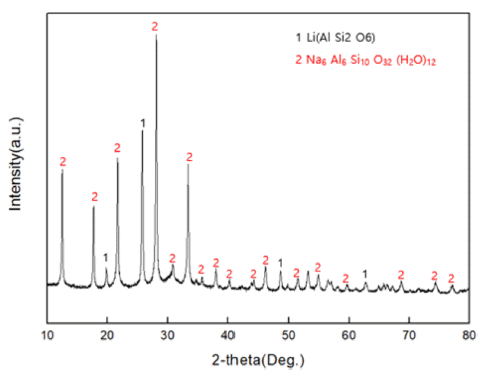
PDF The subject of this study is a zeolite generated as a by-product of recycling LAS (lithium-aluminum-silicate) resources, a kind of glass and ceramic produced by induction. The zeolite by-product is modified into Mg-zeolite using Mg as a cation to absorb Pb, a heavy metal generated from water pollution caused by recent industrial wastewater. An ion-exchange method is used to carry out the modification process, from zeolite byproduct to Mg-zeolite, and simultaneously absorb the Pb in the heavy-metal solution (99.032 mg/L). It is found that the sodium zeolite in the raw material residue can be modified to magnesium zeolite by reacting it with a mixture solution at 1 M concentration for 24 h. As a result, it is found that the residual Pb (0.130 mg/L) in the heavy metal solution is shown to be absorbed by 99.86%, with successful formation of a Mg-modified zeolite.
-
Citations
Citations to this article as recorded by- Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
Rohmat Ismail, Manasye Erlangga, Ari Himawan, Givana Indah Nurul Afiah
Jurnal Pengelolaan Laboratorium Pendidikan.2025; 7(2): 81. CrossRef - Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics
Kinjal J. Shah, Jiacheng Yu, Ting Zhang, Zhaoyang You
Water.2023; 15(6): 1171. CrossRef
- Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
- [English]
- Synthesis of Nanosized Nickel Particle from Spent Cathodic Material Containing Lithium
- Jei-Pil Wang
- J Korean Powder Metall Inst. 2019;26(4):340-344. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.340
- 921 View
- 4 Download
-
 Abstract
Abstract
 PDF
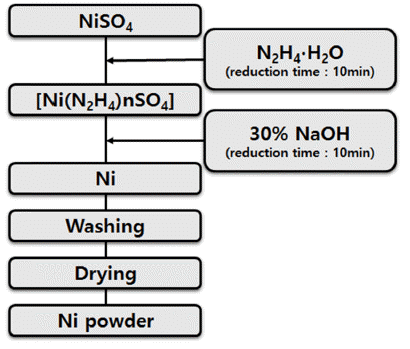
PDF Due to the rapid development of electricity, electronics, information communication, and biotechnology in recent years, studies are actively being conducted on nanopowders as it is required not only for high strengthening but also for high-function powder with electric, magnetic, and optical properties. Nonetheless, studies on nickel nanopowders are rare. In this study of the synthesis of nickel nanoparticles from LiNiO2 (LNO), which is a cathode active material, we have synthesized the nanosized nickel powder by the liquid reduction process of NiSO4 obtained through the leaching and purification of LNO. Moreover, we have studied the reduction reaction rate according to the temperature change of liquid phase reduction and the change of particle size as a function of NaOH addition amount using hydrazine monohydrate (N2H4·H2O) and NaOH.
- [English]
- A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
- Jei-Pil Wang, Jae-Jung Pyo, Se-Ho Ahn, Dong-Hyeon Choi, Byeong-Woo Lee, Dong-Won Lee
- J Korean Powder Metall Inst. 2018;25(4):296-301. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.296
- 3,450 View
- 74 Download
- 11 Citations
-
 Abstract
Abstract
 PDF
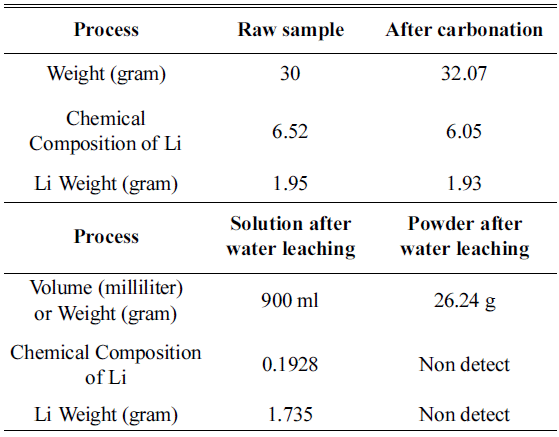
PDF In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered.
-
Citations
Citations to this article as recorded by- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
Amir Hossein Mohammad Zadeh, Spencer Cunnigham, Devon Gray, Sevan Bedrossian, Baian Almusned, Jeffrey Daniel Henderson, Gisele Azimi
Waste Management.2026; 212: 115339. CrossRef - Hydrogen Reduction of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries
Jae-Ho Hwang, Sang-Yeop Lee, So-Yeong Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(3): 34. CrossRef - Reduction Roasting of Cathode Materials of NCM Based Lithium-ion Batteries Using CH4(g)
Jae-Ho Hwang, Sang-Yeop Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(4): 48. CrossRef - High‐Rate Rechargeable Li/SOCl2 Batteries Enabled by Cobalt Phthalocyanine Cathodic Catalysts
Yingxuan Song, Haibo Ouyang, Zhanwei Xu, Zeyang Zhang, Kang Li, Jianfeng Huang, Zhi Li, Tian Wang, Jun Yang
Small.2025;[Epub] CrossRef - Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef - Metals Recovery from Spent Lithium-ion Batteries Cathode Via Hydrogen Reduction-water Leaching-carbothermic or Hydrogen Reduction Process
Tahereh Rostami, Behnam Khoshandam
Mining, Metallurgy & Exploration.2024; 41(3): 1485. CrossRef - Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(5): 923. CrossRef - Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(12): 2000. CrossRef - Environmentally Friendly Recovery of Lithium from Lithium–Sulfur Batteries
Lilian Schwich, Bernd Friedrich
Metals.2022; 12(7): 1108. CrossRef - Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Lilian Schwich, Tom Schubert, Bernd Friedrich
Metals.2021; 11(2): 177. CrossRef - Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem
Jiadong Yu, Quanyin Tan, Jinhui Li
Journal of Cleaner Production.2020; 255: 120269. CrossRef
- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
- [Korean]
- Fabrication of TiC powder by carburization of TiH2 powder
- Hun-Seok Lee, Hyang-Im Seo, Young-Seon Lee, Dong-Jun Lee, Jei-Pil Wang, Dong-Won Lee
- J Korean Powder Metall Inst. 2017;24(1):29-33. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.29

- 672 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Titanium carbide (TiC) powders are successfully synthesized by carburization of titanium hydride (TiH2) powders. The TiH2 powders with size lower than 45 μm (-325 Mesh) are optimally produced by the hydrogenation process, and are mixed with graphite powder by ball milling. The mixtures are then heat-treated in an Ar atmosphere at 800-1200oC for carburization to occur. It has been experimentally and thermodynamically determined that the dehydrogenation, “TiH2 = Ti + H2”, and carburization, “Ti + C = TiC”, occur simultaneously over the reaction temperature range. The unreacted graphite content (free carbon) in each product is precisely measured by acid dissolution and by the filtering method, and it is possible to conclude that the maximal carbon stoichiometry of TiC0.94 is accomplished at 1200°C.
-
Citations
Citations to this article as recorded by- Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef
- Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
- [Korean]
- Study on the Reduction of Forging Oxide Scale using Hydrogen
- Dong-Won Lee, Jung-Yeul Yun, Shun-Myung Shin, In-Soo Kim, Jei-Pil Wang
- J Korean Powder Metall Inst. 2013;20(3):174-179.
- DOI: https://doi.org/10.4150/KPMI.2013.20.3.174

- 837 View
- 18 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF - The study on the fabrication of iron powder from forging scales using hydrogen gas has been conducted on the effect of hydrogen partial pressure, temperature, and reactive time. The mechanism for the reduction of iron oxides was proposed with various steps, and it was found that reduction pattern might be different depending on temperature. The iron content in the scale and reduction ratio of oxygen were both increased with increasing reactive time at 0.1atm of hydrogen partial pressure. On the other hand, for over 30 minutes at 0.5 atm of hydrogen partial pressure, the values were found to be almost same. In the long run, iron metallic powder was obtained with over 90% of iron content and an average size of its powder was observed to be about 100µm.
-
Citations
Citations to this article as recorded by- A Comprehensive Review on Polyaniline/Ferrite Nanocomposites for Gas Sensing Applications
Shreedhara Kondajji Murugendrappa, Ashwini Rayar, Naveen Chikkahanumajja Surendranatha, Thejas Ramakrishnaiah, Prasanna Gunderi Dhananjaya
physica status solidi (a).2025;[Epub] CrossRef - Effects of expanded graphite content on the performance of MgO‐C refractories
Junseong Kim, Seunghwa Jeong, Minsuk Lee, Dong Jae Kang, Hong‐woo Park, Hwa‐In Lee, Dong‐Yeol Yang, Eun Hee Kim, Soonil Lee, Seung‐Cheol Yang, Sang‐Chae Jeon
International Journal of Applied Ceramic Technology.2023; 20(6): 3803. CrossRef - Smithing Processes Based on Hammer Scale Excavated from the Third- to Fourth-Century Ancient Iron-Making Sites of the Korean Peninsula
Dayeon Jung, Heehong Kwon, Namchul Cho
Materials.2022; 15(12): 4188. CrossRef
- A Comprehensive Review on Polyaniline/Ferrite Nanocomposites for Gas Sensing Applications
- [Korean]
- Trend in Research and Development of Recovery of Valuable Metallic Powder from Wasted Batteries
- Shun-Myung Shin, Sung-Ho Joo, Dong-Won Lee, Jung-Yeul Yun, Jei-Pil Wang
- J Korean Powder Metall Inst. 2013;20(1):60-67.
- DOI: https://doi.org/10.4150/KPMI.2013.20.1.060

- 814 View
- 1 Download
- 2 Citations
-
 PDF
PDF -
Citations
Citations to this article as recorded by- A study on Zn recovery from other metals in the spent mixed batteries through a sequence of hydrometallurgical processes
Dong Ju Shin, Sung-Ho Joo, Chang-Hyun Oh, Jei-Pil Wang, Jin-Tae Park, Dong Joon Min, Shun Myung Shin
Environmental Technology.2019; 40(26): 3512. CrossRef - Development of Batch-Type Electric Furnace for Recovery of Valuable Materials from Spent Batteries
Shun Myung Shin, Dong Won Lee, Jung Yeul Yun, Byung Ho Jung, Jei Pil Wang
Applied Mechanics and Materials.2014; 607: 197. CrossRef
- A study on Zn recovery from other metals in the spent mixed batteries through a sequence of hydrometallurgical processes
- [English]
- Oxidation Study on the Fabrication of Fe-36Ni Oxide Powder from Its Scrap
- Jung Yeul Yun, Man Ho Park, Sangsun Yang, Dong-Won Lee, Jei-Pil Wang
- J Korean Powder Metall Inst. 2013;20(1):48-52.
- DOI: https://doi.org/10.4150/KPMI.2013.20.1.048

- 482 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF - A study of oxidation kinetic of Fe-36Ni alloy has been investigated using thermogravimetric apparatus (TGA) in an attempt to define the basic mechanism over a range of temperature of 400 to 1000°C and finally to fabricate its powder. The oxidation rate was increased with increasing temperature and oxidation behavior of the alloy followed a parabolic rate law at elevated temperature. Temperature dependence of the reaction rate was determined with Arrhenius-type equation and activation energy was calculated to be 106.49 kJ/mol. Based on the kinetic data and micro-structure examination, oxidation mechanism was revealed that iron ions and electrons might migrate outward along grain boundaries and oxygen anion diffused inward through a spinel structure, (Ni,Fe)_3O_4.
- [Korean]
- Extraction of Vanadium Powder by Metallothermic Reduction
- Dong-Won Lee, Sang-Hyun Heo, Jong-Taek Yeom, Jei-Pil Wang
- J Korean Powder Metall Inst. 2013;20(1):43-47.
- DOI: https://doi.org/10.4150/KPMI.2013.20.1.043

- 798 View
- 1 Download
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF - The extraction of metallic pure vanadium powder from raw oxide has been tried by Mg-reduction. In first stage, V_2O_5 powders as initial raw material was reduced by hydrogen gas into V_2O_3 phase. V_2O_3 powder was reduced in next stage by magnesium gas at 1,073K for 24 hours. After reduction reaction, the MgO component mixed with reduced vanadium powder were dissolved and removed fully in 10% HCl solution for 5 hours at room temperature. The oxygen content and particle size of finally produced vanadium powders were 0.84 wt% and 1 µm, respectively
-
Citations
Citations to this article as recorded by- Preparation of tantalum metal powder by magnesium gas reduction of tantalum pentoxide with different initial particle size
Seon-Min Hwang, Su-Jin Park, Jei-Pil Wang, Yong-Ho Park, Dong-Won Lee
International Journal of Refractory Metals and Hard Materials.2021; 100: 105620. CrossRef - Metallic Niobium Powder Reduced by Atmospheric Magnesium Gas with Niobium Pentoxide Powder
Su-Jin Park, Seon-Min Hwang, Jei-pil Wang, Young-Guk Son, Dong-Won Lee
MATERIALS TRANSACTIONS.2021; 62(1): 34. CrossRef - Fabrication of Metallic Tantalum Powder by Magnesium-gas Reduction of Tantalum Oxide
Dong-Won Lee
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 390. CrossRef - Effect of magnesium on the phase equilibria in magnesio-thermic reduction of Nb2O5
Kyunsuk Choi, Hanshin Choi, Hyunwoong Na, Il Sohn
Materials Letters.2016; 183: 151. CrossRef
- Preparation of tantalum metal powder by magnesium gas reduction of tantalum pentoxide with different initial particle size
TOP
 KPMI
KPMI


 First
First Prev
Prev


