Search
- Page Path
- HOME > Search
- [Korean]
- A Study on Morphology Control of (Ga1-xZnx)(N1-xOx) Nanofibers according to the Composition and Crystallinity of Oxide Nanofibers Synthesized by Electrospinning
- Jeong Hyun Kim, Sung-Tag Oh, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(3):259-266. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.259
- 625 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
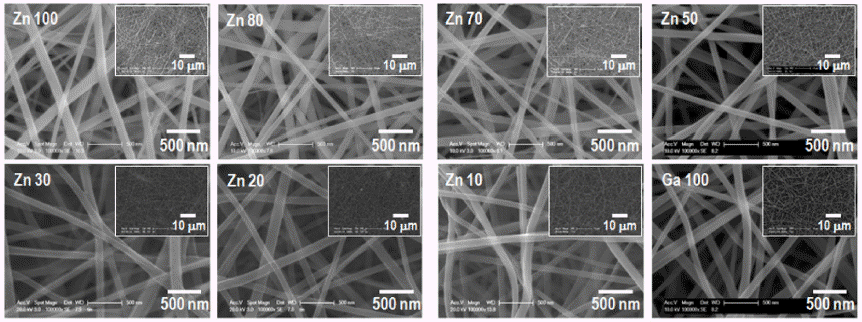
PDF The (Ga1-xZnx)(N1-xOx) solid solution is attracting extensive attention for photocatalytic water splitting and wastewater treatment owing to its narrow and controllable band gap. To optimize the photocatalytic performance of the solid solution, the key points are to decrease its band gap and recombination rate. In this study, (Ga1-xZnx)(N1-xOx) nanofibers with various Zn fractions are prepared by electrospinning followed by calcination and nitridation. The effect of the composition and crystallinity of electrospun oxide nanofibers on the morphology and optical properties of the obtained solid-solution nanofibers are systematically investigated. The results show that the final shape of the (Ga1-xZnx) (N1-xOx) material is greatly affected by the crystallinity of the oxide nanofibers before nitridation. The photocatalytic properties of (Ga1-xZnx)(N1-xOx) with different Ga:Zn atomic ratios are investigated by studying the degradation of rhodamine B under visible light irradiation.
-
Citations
Citations to this article as recorded by- Fabrication of Nanowire by Electrospinning Process Using Nickel Oxide Particle Recovered from MLCC
Haein Shin, Jongwon Bae, Minsu Kang, Kun-Jae Lee
journal of Korean Powder Metallurgy Institute.2023; 30(6): 502. CrossRef
- Fabrication of Nanowire by Electrospinning Process Using Nickel Oxide Particle Recovered from MLCC
- [Korean]
- Synthesis and Optical Property of (GaN)1-x(ZnO)x Nanoparticles Using an Ultrasonic Spray Pyrolysis Process and Subsequent Chemical Transformation
- Jeong Hyun Kim, Cheol-Hui Ryu, Myungjun Ji, Yomin Choi, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(2):143-149. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.143
- 764 View
- 2 Download
-
 Abstract
Abstract
 PDF
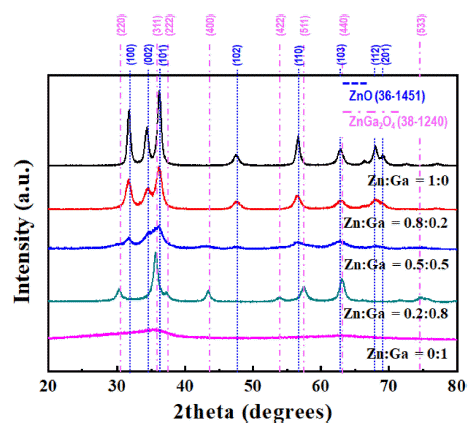
PDF In this study, (GaN)1-x(ZnO)x solid solution nanoparticles with a high zinc content are prepared by ultrasonic spray pyrolysis and subsequent nitridation. The structure and morphology of the samples are investigated by X-ray diffraction (XRD), field-emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The characterization results show a phase transition from the Zn and Ga-based oxides (ZnO or ZnGa2O4) to a (GaN)1-x (ZnO)x solid solution under an NH3 atmosphere. The effect of the precursor solution concentration and nitridation temperature on the final products are systematically investigated to obtain (GaN)1-x(ZnO)x nanoparticles with a high Zn concentration. It is confirmed that the powder synthesized from the solution in which the ratio of Zn and Ga was set to 0.8:0.2, as the initial precursor composition was composed of about 0.8-mole fraction of Zn, similar to the initially set one, through nitriding treatment at 700°C. Besides, the synthesized nanoparticles exhibited the typical XRD pattern of (GaN)1-x(ZnO)x, and a strong absorption of visible light with a bandgap energy of approximately 2.78 eV, confirming their potential use as a hydrogen production photocatalyst.
- [Korean]
- Effect of Calcination Temperature on the Microstructure and Photocatalytic Activity of Electrospun BiVO4 Nanofiber
- Myeongjun Ji, Jeong Hyun Kim, Cheol-Hui Ryu, Yun Taek Ko, Young-In Lee
- J Korean Powder Metall Inst. 2020;27(3):226-232. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.226
- 1,182 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
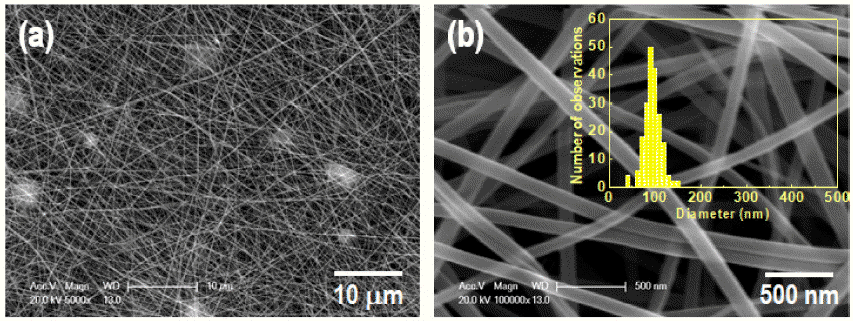
PDF Bismuth vanadate (BiVO4) is considered a potentially attractive candidate for the visible-light-driven photodegradation of organic pollutants. In an effort to enhance their photocatalytic activities, BiVO4 nanofibers with controlled microstructures, grain sizes, and crystallinities are successfully prepared by electrospinning followed by a precisely controlled heat treatment. The structural features, morphologies, and photo-absorption performances of the asprepared samples are systematically investigated and can be readily controlled by varying the calcination temperature. From the physicochemical analysis results of the synthesized nanofiber, it is found that the nanofiber calcines at a lower temperature, shows a smaller crystallite size, and lower crystallinity. The photocatalytic degradation of rhodamine-B (RhB) reveals that the photocatalytic activity of the BiVO4 nanofibers can be improved by a thermal treatment at a relatively low temperature because of the optimization of the conflicting characteristics, crystallinity, crystallite size, and microstructure. The photocatalytic activity of the nanofiber calcined at 350°C for the degradation of RhB under visible-light irradiation exhibits a greater photocatalytic activity than the nanofibers synthesized at 400°C and 450°C.
-
Citations
Citations to this article as recorded by- Design, synthesis, and characterization of a porous ceramic-supported CeO2 nanocatalyst for CO -free H2 evolution
Jimin Lee, Minseob Lim, Tae Sung Kim, Kee-Ryung Park, Jong-Sik Lee, Hong-Baek Cho, Joo Hyun Park, Yong-Ho Choa
Applied Surface Science.2021; 548: 149198. CrossRef
- Design, synthesis, and characterization of a porous ceramic-supported CeO2 nanocatalyst for CO -free H2 evolution
TOP
 KPMI
KPMI


 First
First Prev
Prev


