Search
- Page Path
- HOME > Search
- [Korean]
- Effect of Abnormal Grain Growth on Ionic Conductivity in LATP
- Hyungik Choi, Yoonsoo Han
- J Powder Mater. 2024;31(1):23-29. Published online February 28, 2024
- DOI: https://doi.org/10.4150/KPMI.2024.31.1.23
- 2,925 View
- 66 Download
- 2 Citations
-
 PDF
PDF -
Citations
Citations to this article as recorded by- Temperature-dependent microstructural evolution in a compositionally complex solid electrolyte: The role of a grain boundary transition
Shu-Ting Ko, Chaojie Du, Huiming Guo, Hasti Vahidi, Jenna L. Wardini, Tom Lee, Yi Liu, Jingjing Yang, Francisco Guzman, Timothy J. Rupert, William J. Bowman, Shen J. Dillon, Xiaoqing Pan, Jian Luo
Journal of Advanced Ceramics.2025; 14(3): 9221047. CrossRef - Effect of bimodal particle size distribution on Li1.5Al0.5Ti1.5(PO4)3 solid electrolytes: Microstructures and electrochemical properties
Gi Jeong Kim, Yeon Hee Kim, Seul Ki Choi, Jong Won Bae, Kun-Jae Lee, Minho Yang
Powder Technology.2025; 466: 121407. CrossRef
- Temperature-dependent microstructural evolution in a compositionally complex solid electrolyte: The role of a grain boundary transition
- [Korean]
- Effect of Chelating Agent on Li1.5Al0.5Ti1.5(PO4)3 Particles by Sol-gel Method and Densification
- SungJoon Ryu, Seul Ki Choi, Jong Ho Won, MinHo Yang
- J Powder Mater. 2023;30(5):394-401. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.394
- 1,763 View
- 48 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
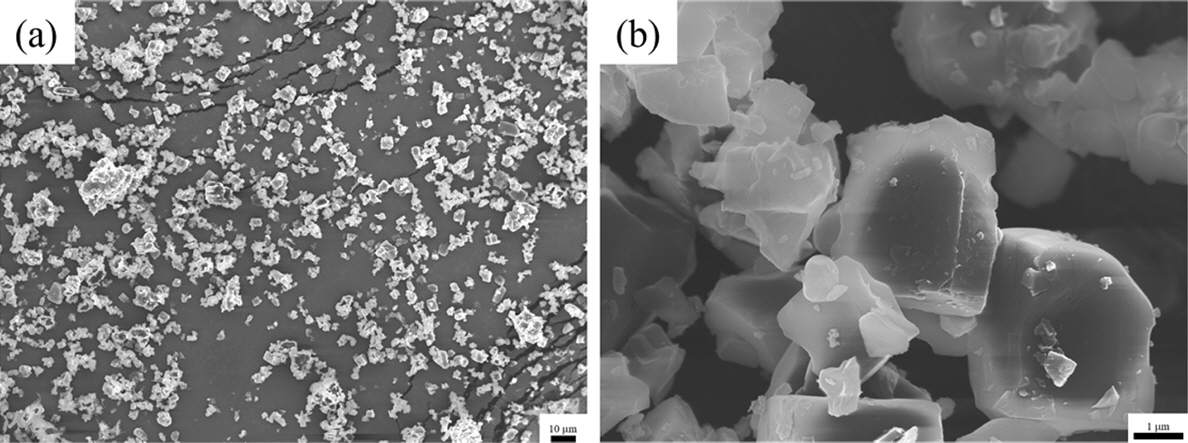
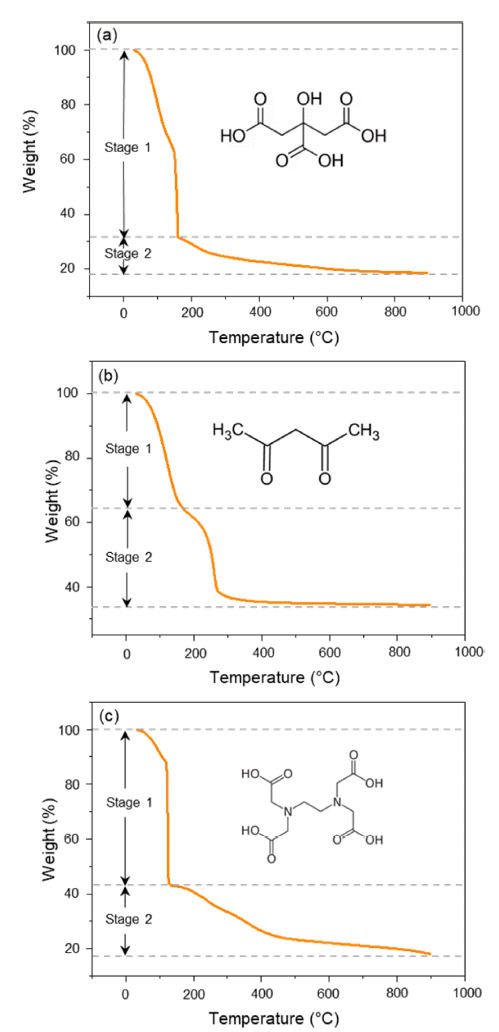
PDF Li1.5Al0.5Ti1.5(PO4)3 (LATP) is considered to be one of the promising solid-state electrolytes owing to its excellent chemical and thermal stability, wide potential range (~5.0 V), and high ionic conductivity (~10-4 S/cm). LATP powders are typically prepared via the sol-gel method by adding and mixing nitrate or alkoxide precursors with chelating agents. Here, the thermal properties, crystallinity, density, particle size, and distribution of LATP powders based on chelating agents (citric acid, acetylacetone, EDTA) are compared to find the optimal conditions for densely sintered LATP with high purity. In addition, the three types of LATP powders are utilized to prepare sintered solid electrolytes and observe the microstructure changes during the sintering process. The pyrolysis onset temperature and crystallization temperature of the powder samples are in the order AC-LATP > CA-LATP > ED-LATP, and the LATP powder utilizing citric acid exhibits the highest purity, as no secondary phase other than LiTi2PO4 phase is observed. LATP with citric acid and acetylacetone has a value close to the theoretical density (2.8 g/cm3) after sintering. In comparison, LATP with EDTA has a low sintered density (2.2 g/cm3) because of the generation of many pores after sintering.
-
Citations
Citations to this article as recorded by- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef
- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
- [Korean]
- A Study on the Microstructures and Ionic Conductivity of Li1.3Al0.3Ti1.7(PO4)3 with Different Synthesis Routes
- Seul Ki Choi, Jeawon Choi, MinHo Yang
- J Powder Mater. 2023;30(2):107-115. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.107
- 2,125 View
- 48 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
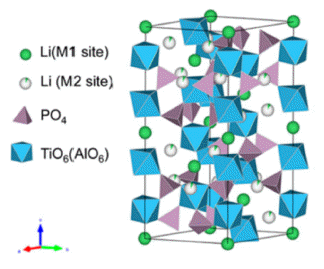
PDF Li1.3Al0.3Ti1.7(PO4)3(LATP) is considered a promising material for all-solid-state lithium batteries owing to its high moisture stability, wide potential window (~6 V), and relatively high ion conductivity (10-3–10-4 S/cm). Solid electrolytes based on LATP are manufactured via sintering, using LATP powder as the starting material. The properties of the starting materials depend on the synthesis conditions, which affect the microstructure and ionic conductivity of the solid electrolytes. In this study, we synthesize the LATP powder using sol-gel and co-precipitation methods and characterize the physical properties of powder, such as size, shape, and crystallinity. In addition, we have prepared a disc-shaped LATP solid electrolyte using LATP powder as the starting material. In addition, X-ray diffraction, scanning electron microscopy, and electrochemical impedance spectroscopic measurements are conducted to analyze the grain size, microstructures, and ion conduction properties. These results indicate that the synthesis conditions of the powder are a crucial factor in creating microstructures and affecting the conduction properties of lithium ions in solid electrolytes.
-
Citations
Citations to this article as recorded by- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
Gwanhee Song, Bojoong Kim, Inkook Hwang, Jiwon Kim, Jinmo Kim, Chang-Bun Yoon
Materials.2025; 18(3): 609. CrossRef
- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
TOP
 KPMI
KPMI


 First
First Prev
Prev


