Search
- Page Path
- HOME > Search
- [Korean]
- Recent Developments in Quantum Dot Patterning Technology for Quantum Dot Display
- Yeong Jun Jin, Kyung Jun Jung, Jaehan Jung
- J Powder Mater. 2024;31(2):169-179. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00073
- 7,008 View
- 155 Download
-
 Abstract
Abstract
 PDF
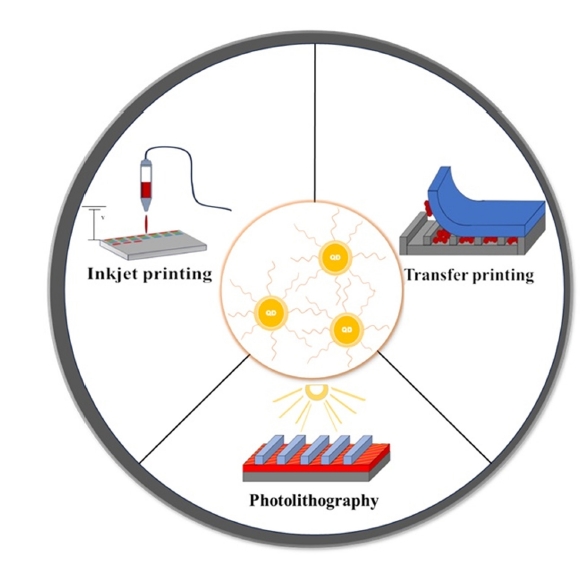
PDF - Colloidal quantum dot (QDs) have emerged as a crucial building block for LEDs due to their size-tunable emission wavelength, narrow spectral line width, and high quantum efficiency. Tremendous efforts have been dedicated to improving the performance of quantum dot light-emitting diodes (QLEDs) in the past decade, primarily focusing on optimization of device architectures and synthetic procedures for high quality QDs. However, despite these efforts, the commercialization of QLEDs has yet to be realized due to the absence of suitable large-scale patterning technologies for high-resolution devices., This review will focus on the development trends associated with transfer printing, photolithography, and inkjet printing, and aims to provide a brief overview of the fabricated QLED devices. The advancement of various quantum dot patterning methods will lead to the development of not only QLED devices but also solar cells, quantum communication, and quantum computers.
- [Korean]
- Aqueous Synthesis and Luminescent Characteristics of Cu:ZnSe Quantum Dots by Internal Doping Method
- Geum Ji Back, Hyun Seon Hong
- J Powder Mater. 2022;29(5):370-375. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.370
- 852 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
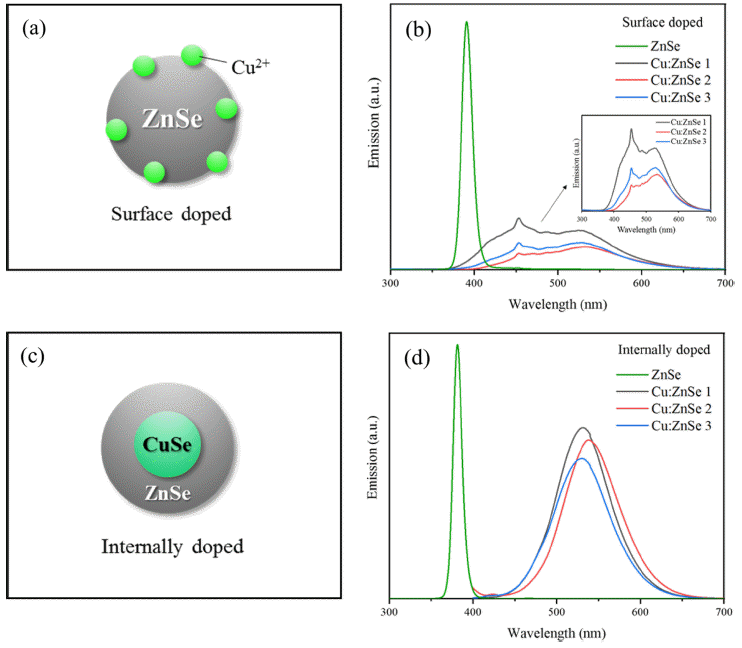
PDF Cu-doped ZnSe quantum dots were successfully synthesized in an aqueous solution using an internal doping method. The effects of ligand type, CuSe synthesis temperature, and heating time on Cu-doped ZnSe synthesis were systematically investigated. Of MPA, GSH, TGA, and NAC used as ligands, MPA was the optimal ligand as determined by PL spectrum analysis. In addition, the emission wavelength was found to depend on the synthesis temperature of the internal doping core of CuSe. As the temperature increased, the doping of Cu2+ was enhanced, and the emission wavelength band was redshifted; accordingly, the emission peaks moved from blue to green (up to 550 nm). Thus, the synthesis of Cu:ZnSe using internal doping in aqueous solutions is a potential method for ecomanufacturing of colortuned ZnSe quantum dots for display applications.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
- [Korean]
- Synthesis of Highly Dispersible Metal Nanoparticles in P3HT:PCBM Layers and Their Effects on the Performance of Polymer Solar Cells
- Min-Ji Kim, Gyu-Chae Choi, Young-Kuk Kim, Yang-Do Kim, Youn-Kyoung Baek
- J Korean Powder Metall Inst. 2014;21(3):179-184. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.179

- 475 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF In this study, we prepare polymer solar cells incorporating organic ligand-modified Ag nanoparticles (OAgNPs) highly dispersed in the P3HT:PCBM layer. Ag nanoparticles decorated with water-dispersible ligands (WAgNPs) were also utilized as a control sample. The existence of the ligands on the Ag surface was confirmed by FTIR spectra. Metal nanoparticles with different surface chemistries exhibited different dispersion tendencies. O-AgNPs were highly dispersed even at high concentrations, whereas W-AgNPs exhibited significant aggregation in the polymer layer. Both dispersion and blending concentration of the Ag nanoparticles in P3HT:PCBM matrix had critical effects on the device performance as well as light absorption. The significant changes in short-circuit current density (JSC) of the solar cells seemed to be related to the change in the polymer morphology according to the concentration of AgNPs introduced. These findings suggested the importance of uniform dispersion of plasmonic metal nanoparticles and their blending concentration conditions in order to boost the solar cell performance.
TOP
 KPMI
KPMI


 First
First Prev
Prev


