Search
- Page Path
- HOME > Search
- [Korean]
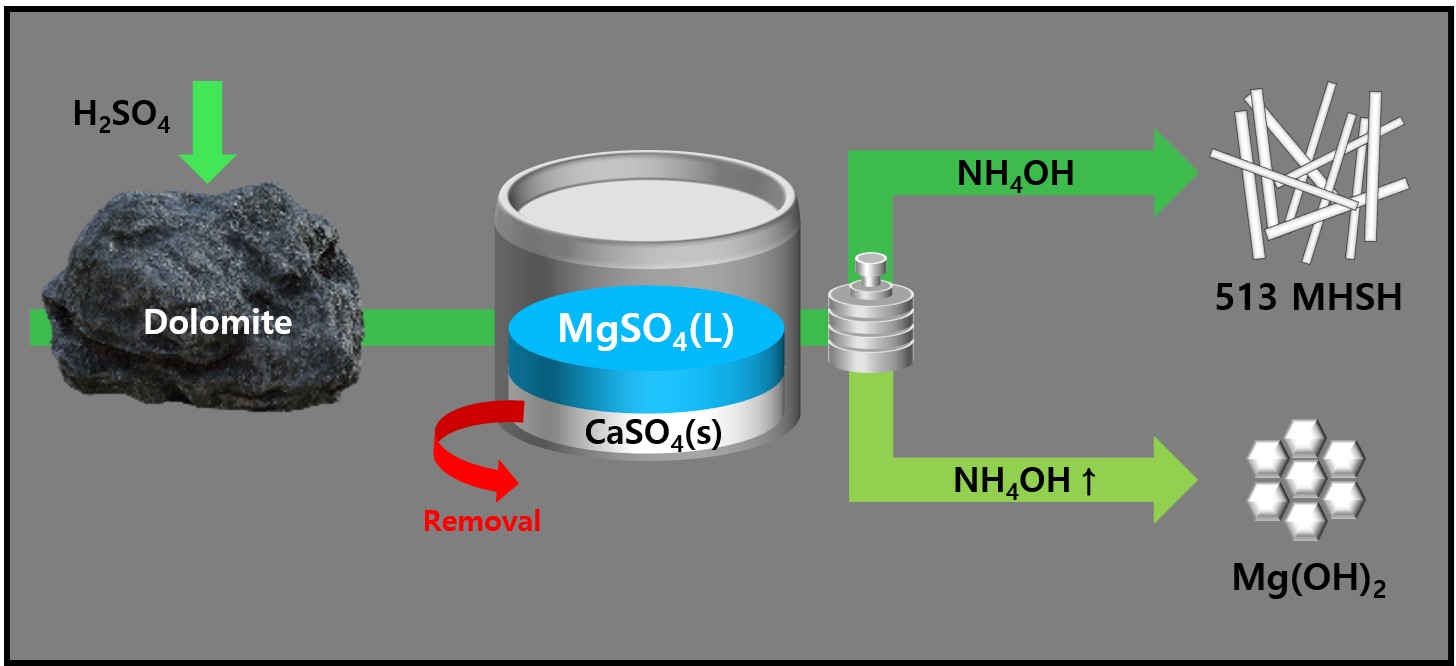
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
- Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
- J Powder Mater. 2025;32(5):399-405. Published online October 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00227
- 372 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF - 513 magnesium hydroxide sulfate hydrate (MHSH) and Mg(OH)₂ were synthesized by controlling the pH and concentration using a domestic resource, dolomite (CaMg(CO3)2), as the raw material. The MgSO₄ was extracted by treating dolomite with sulfuric acid under various conditions. Hexagonal plate-shaped Mg(OH)₂ and needle-like 513 MHSH were synthesized under the hydrothermal condition. The morphology of the synthesized materials was controlled by adjusting the pH (SO42-/OH- ratio) and hydrothermal reaction time. As the pH of the solution increased, the formation of plate-like structures became dominant, whereas lower pH values (higher SO42- concentration) led to needle-like forms. The results of the 513 MHSH, which was synthesized using reagents and sea bittern, are consistent with the synthesis conditions, and we observed changes in the length and aspect ratio of the needle-shaped structure in response to adjusting the hydrothermal reaction time.
- [Korean]
- Effect of H2SO4 and Reaction Time on Synthesis of 5Mg(OH)2∙MgSO4∙3H2O Whiskers using Hydrothermal Reaction
- Areum Choi, Nuri Oh, YooJin Kim
- J Korean Powder Metall Inst. 2020;27(5):401-405. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.401

- 1,003 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Magnesium hydroxide sulfate hydrate (MHSH) whiskers were synthesized via a hydrothermal reaction by using MgO as the reactant as well as the acid solution. The effects of the H2SO4 amount and reaction time at the same temperature were studied. In general, MHSH whiskers were prepared using MgSO4 in aqueous ammonia. In this work, to reduce the formation of impurities and increase the purity of MHSH, we employed a synthesis technique that did not require the addition of a basic solution. Furthermore, the pH value, which was controlled by the H2SO4 amount, acted as an important factor for the formation of high-purity MHSH. MgO was used as the raw material because it easily reacts in water and forms Mg+ and MgOH+ ions that bind with SO4 2- ions to produce MHSH. Their morphologies and structures were determined using X-ray diffraction (XRD) and scanning electron microscopy (SEM).
-
Citations
Citations to this article as recorded by- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
Journal of Powder Materials.2025; 32(5): 399. CrossRef - Study of SiO2 coating and carboxylic surface-modification on Mg-based inorganic fiber by one-step reflux reaction
Minsol Park, Areum Choi, Seiki Kim, Wooyoung Shim, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(6): 869. CrossRef - Effect of sulfate ion on synthesis of 5 Mg(OH)2·MgSO4·3H2O whiskers using non-hydrothermal method with acid catalyst
Areum Choi, Nuri Oh, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(2): 224. CrossRef
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
TOP
 KPMI
KPMI


 First
First Prev
Prev


