Search
- Page Path
- HOME > Search
- [Korean]
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- Minsu Kim, Hansung Lee, Byungmin Ahn
- J Powder Mater. 2023;30(6):478-483. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.478
- 1,152 View
- 15 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
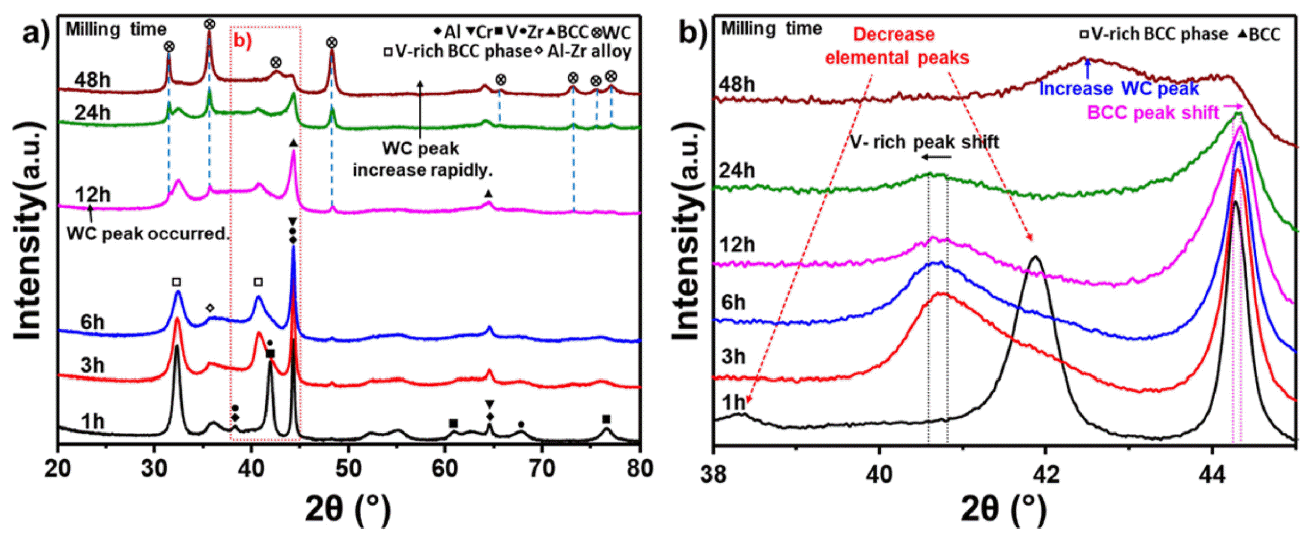
PDF High-entropy alloys (HEAs) are characterized by having five or more main elements and forming simple solids without forming intermetallic compounds, owing to the high entropy effect. HEAs with these characteristics are being researched as structural materials for extreme environments. Conventional refractory alloys have excellent hightemperature strength and stability; however, problems occur when they are used extensively in a high-temperature environment, leading to reduced fatigue properties due to oxidation or a limited service life. In contrast, refractory entropy alloys, which provide refractory properties to entropy alloys, can address these issues and improve the hightemperature stability of the alloy through phase control when designed based on existing refractory alloy elements. Refractory high-entropy alloys require sufficient milling time while in the process of mechanical alloying because of the brittleness of the added elements. Consequently, the high-energy milling process must be optimized because of the possibility of contamination of the alloyed powder during prolonged milling. In this study, we investigated the hightemperature oxidation behavior of refractory high-entropy alloys while optimizing the milling time.
-
Citations
Citations to this article as recorded by- Tailored high-temperature oxidation behavior and nanomechanical properties of Al0.75VCrZrNb lightweight refractory high-entropy alloys
Hansung Lee, Hwi Geun Yu, Reliance Jain, Man Mohan, Younggeon Lee, Sheetal Kumar Dewangan, Byungmin Ahn
International Journal of Refractory Metals and Hard Materials.2026; 135: 107507. CrossRef - Simultaneous enhancement of strength and ductility of Al matrix composites enabled by submicron-sized high-entropy alloy phases
Chahee Jung, Seungin Nam, Hansol Son, Juyeon Han, Jaewon Jeong, Hyokyung Sung, Hyoung Seop Kim, Seok Su Sohn, Hyunjoo Choi
Journal of Materials Research and Technology.2024; 33: 1470. CrossRef
- Tailored high-temperature oxidation behavior and nanomechanical properties of Al0.75VCrZrNb lightweight refractory high-entropy alloys
- [Korean]
- Oxidation Behaviors and Degradation Properties of Aluminide Coated Stainless Steel at High Temperature
- Cheol Hong Hwang, Hyo Min Lee, Jeong Seok Oh, Dong Hyeon Hwang, Yu Seok Hwang, Jong Won Lee, Jeong Mook Choi, Joon Sik Park
- J Korean Powder Metall Inst. 2021;28(5):396-402. Published online October 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.5.396
- 722 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
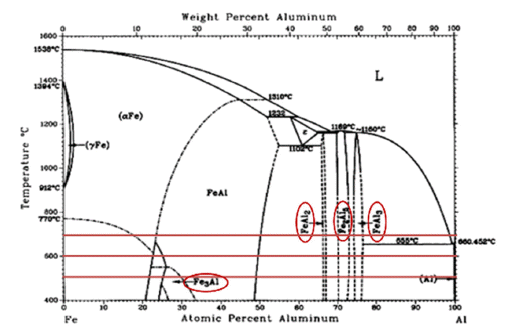
PDF Stainless steel, a type of steel used for high-temperature parts, may cause damage when exposed to high temperatures, requiring additional coatings. In particular, the Cr2O3 product layer is unstable at 1000°C and higher temperatures; therefore, it is necessary to improve the oxidation resistance. In this study, an aluminide (Fe2Al5 and FeAl3) coating layer was formed on the surface of STS 630 specimens through Al diffusion coatings from 500°C to 700°C for up to 25 h. Because the coating layers of Fe2Al5 and FeAl3 could not withstand temperatures above 1200°C, an Al2O3 coating layer is deposited on the surface through static oxidation treatment at 500°C for 10 h. To confirm the ablation resistance of the resulting coating layer, dynamic flame exposure tests were conducted at 1350°C for 5–15 min. Excellent oxidation resistance is observed in the coated base material beneath the aluminide layer. The conditions of the flame tests and coating are discussed in terms of microstructural variations.
-
Citations
Citations to this article as recorded by- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
C. Hwang, J. Park, J. Oh, D. Han, S. Lee, K. Shin, J. Choi, K. P. Shinde, J. S. Park
Metals and Materials International.2023; 29(7): 1855. CrossRef
- Thermal Stability and Degradation Properties of Aluminide Coated and Uncoated Ti-6Al-4V Alloys Exposed to High Temperature Flame
- [Korean]
- High Temperature Oxidation Behavior of 316L Austenitic Stainless Steel Manufactured by Laser Powder Bed Fusion Process
- Yu-Jin Hwang, Dong-Yeol Wi, Kyu-Sik Kim, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2021;28(2):110-119. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.110
- 1,139 View
- 26 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
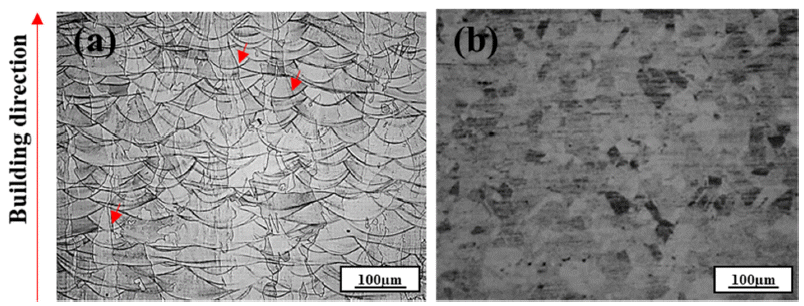
PDF In this study, the high-temperature oxidation properties of austenitic 316L stainless steel manufactured by laser powder bed fusion (LPBF) is investigated and compared with conventional 316L manufactured by hot rolling (HR). The initial microstructure of LPBF-SS316L exhibits a molten pool ~100 μm in size and grains grown along the building direction. Isotropic grains (~35 μm) are detected in the HR-SS316L. In high-temperature oxidation tests performed at 700°C and 900°C, LPBF-SS316L demonstrates slightly superior high-temperature oxidation resistance compared to HR-SS316L. After the initial oxidation at 700°C, shown as an increase in weight, almost no further oxidation is observed for both materials. At 900°C, the oxidation weight displays a parabolic trend and both materials exhibit similar behavior. However, at 1100°C, LPBF-SS316L oxidizes in a parabolic manner, but HR-SS316L shows a breakaway oxidation behavior. The oxide layers of LPBF-SS316L and HR-SS316L are mainly composed of Cr2O3, Febased oxides, and spinel phases. In LPBF-SS316L, a uniform Cr depletion region is observed, whereas a Cr depletion region appears at the grain boundary in HR-SS316L. It is evident from the results that the microstructure and the hightemperature oxidation characteristics and behavior are related.
-
Citations
Citations to this article as recorded by- Retention factor-based constitutive model of high-strength austenitic A4–80 bolts after fire exposure
Hui Wang, Bo Yang, Tao Sun, Weilai Yao, Wei Jiang
Journal of Constructional Steel Research.2025; 235: 109930. CrossRef - Study of structural stability at high temperature of pseudo-single tube with double layer as an alternative method for accident-tolerant fuel cladding
Jong Woo Kim, Hyeong Woo Min, Jaehwan Ko, Yonghee Kim, Young Soo Yoon
Journal of Nuclear Materials.2022; 566: 153800. CrossRef
- Retention factor-based constitutive model of high-strength austenitic A4–80 bolts after fire exposure
- [Korean]
- The Effect of Oxides Additives on Anti-corrosion Properties of Sintered 316L Stainless Steel
- Jong-Pil Lee, Ji-Hyun Hong, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2015;22(4):271-277. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.271

- 578 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF As wrought stainless steel, sintered stainless steel (STS) has excellent high-temperature anti-corrosion even at high temperature of 800ºC and exhibit corrosion resistance in air. The oxidation behavior and oxidation mechanism of the sintered 316L stainless was reported at the high temperature in our previous study. In this study, the effects of additives on high-temperature corrosion resistances were investigated above 800ºC at the various oxides (SiO2, Al2O3, MgO and Y2O3) added STS respectively as an oxidation inhibitor. The morphology of the oxide layers were observed by SEM and the oxides phase and composition were confirmed by XRD and EDX. As a result, the weight of STS 316L sintered body increased sharply at 1000oC and the relative density of specimen decreased as metallic oxide addition increased. Compared with STS 316L sintered parts, weight change ratio corresponding to different oxidation time at 900oC and 1000oC, decreased gradually with the addition of metallic oxide. The best corrosion resistance properties of STS could be improved in case of using Y2O3. The oxidation rate was diminished dramatically by suppression the peeling on oxide layers at Y2O3 added sintered stainless steel.
- [Korean]
- The Effects of Composition and Microstructure Variation on the Oxidation Characteristics of Stainless Steels Manufactured by Powder Metallurgy Method
- Jong-Pil Lee, Ji-Hyun Hong, Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2015;22(1):52-59. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.52

- 1,264 View
- 11 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF As well-known wrought stainless steel, sintered stainless steel (STS) has excellent high-temperature anticorrosion even at high temperature of 800°C, and exhibits good corrosion resistance in air. However, when temperature increases above 900°C, the corrosion resistance of STS begins to deteriorate and dramatically decreases. In this study, the effects of phase and composition of STS on high-temperature corrosion resistances are investigated for STS 316L, STS 304 and STS 434L above 800°C. The morphology of the oxide layers are observed. The oxides phase and composition are identified using X-ray diffractometer and energy dispersive spectroscopy. The results demonstrate that the best corrosion resistance of STS could be improved to that of 434L. The poor corrosion resistance of the austenitic stainless steels is due to the fact that NiFe2O4 oxides forming poor adhesion between the matrix and oxide film increase the oxidation susceptibility of the material at high temperature.
-
Citations
Citations to this article as recorded by- The effect of different turbulent flow on failure behavior in secondary loop of the pressurized water reactor
Y. Hu, L. Zhao, Y.H. Lu, T. Shoji
Nuclear Engineering and Design.2020; 368: 110812. CrossRef
- The effect of different turbulent flow on failure behavior in secondary loop of the pressurized water reactor
TOP
 KPMI
KPMI


 First
First Prev
Prev


