Search
- Page Path
- HOME > Search
- [Korean]
- Mn-doping Effect on the Blackness and NIR Reflectance of Fe2O3 Cool Pigments
- Jin Soo Hwang, Kyeong Youl Jung
- J Korean Powder Metall Inst. 2021;28(1):38-43. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.38

- 564 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF A high NIR-reflective black pigment is developed by Mn doping of Fe2O3. The pigment powders are prepared by spray pyrolysis, and the effect of the Mn concentration on the blackness and optical properties is investigated. Mn doping into the crystal lattice of α-Fe2O3 is found to effectively change the powder color from red to black, lowering the NIR reflectance compared to that of pure Fe2O3. The pigment doped with 10% Mn, i.e., Fe1.8Mn0.2O3, exhibits a black color with an optical bandgap of 1.3 eV and a Chroma value of 1.14. The NIR reflectance of the prepared Fe1.8Mn0.2O3 black pigment is 2.2 times higher than that of commercially available carbon black, and this material is proven to effectively work as a cool pigment in a temperature rise experiment under near-infrared illumination.
- [Korean]
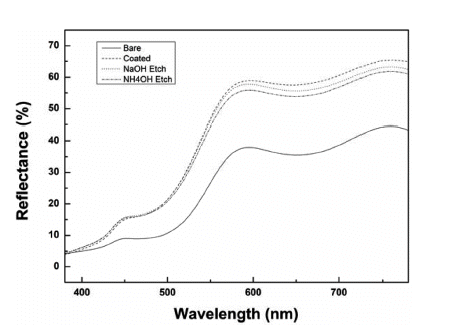
- Study of Color Evolution by Silica Coating and Etching based Morphological Control of α-FeOOH
- NaRi Lee, Ri Yu, YooJin Kim
- J Korean Powder Metall Inst. 2018;25(5):379-383. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.379
- 854 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Silica is used in shell materials to minimize oxidation and aggregation of nanoparticles. Particularly, porous silica has gained attention because of its performance in adsorption, catalysis, and medical applications. In this study, to investigate the effect of the density of the silica coating layer on the color of the pigment, we arbitrarily change the structure of a silica layer using an etchant. We use NaOH or NH4OH to etch the silica coating layer. First, we synthesize α-FeOOH for a length of 400 nm and coat it with TEOS to fabricate particles with a 50 nm coating layer. The coating thickness is then adjusted to 30–40 nm by etching the silica layer for 5 h. Four different shapes of α-FeOOH with different colors are measured using UV–vis light. From the color changes of the four different shapes of α-FeOOH features during coating or etching, the
L * value is observed to increase and brighten the overall color, and theb * value increases to impart a clear yellow color to the pigment. The brightest yellow color was that coated with silica; if the sample is etched with NaOH or NH4OH, theb * value can be controlled to study the yellow colors.-
Citations
Citations to this article as recorded by- Trend of Ceramic Nano Pigments
Ri Yu, YooJin Kim
Ceramist.2019; 22(3): 256. CrossRef
- Trend of Ceramic Nano Pigments
- [Korean]
- Preparation of Nanosized Gd2O3:Eu3+ Red Phosphor Coated on Mica Flake and Its Luminescent Property
- Se-Min Ban, Jeong Min Park, Kyeong Youl Jung, Byung-Ki Choi, Kwang-Jung Kang, Myung Chang Kang, Dae-Sung Kim
- J Korean Powder Metall Inst. 2017;24(6):457-463. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.457

- 634 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Nanosized Gd2O3:Eu3+ red phosphor is prepared using a template method from metal salt impregnated into a crystalline cellulose and is dispersed using a bead mill wet process. The driving force of the surface coating between Gd2O3:Eu3+ and mica is induced by the Coulomb force. The red phosphor nanosol is effectively coated on mica flakes by the electrostatic interaction between positively charged Gd2O3:Eu3+ and negatively charged mica above pH 6. To prepare Gd2O3:Eu3+-coated mica (Gd2O3:Eu/mica), the coating conditions are optimized, including the stirring temperature, pH, calcination temperature, and coating amount (wt%) of Gd2O3:Eu3+. In spite of the low luminescence of the Gd2O3:Eu/mica, the luminescent property is recovered after calcination above 600°C and is enhanced by increasing the Gd2O3:Eu3+ coating amount. The Gd2O3:Eu/mica is characterized using X-ray diffraction, field emission scanning electron microscopy, zeta potential measurements, and fluorescence spectrometer analysis.
-
Citations
Citations to this article as recorded by- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
Mahboob Ullah, Se-Min Ban, Dae-Sung Kim
Optical Materials.2019; 97: 109366. CrossRef
- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
- [Korean]
- Synthesis and Characterization of Brilliant Yellow Color Pigments using α-FeOOH Nanorods
- JiYeon Yun, Ri Yu, YooJin Kim
- J Korean Powder Metall Inst. 2016;23(6):453-457. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.453

- 377 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this work, we synthesize brilliant yellow color α-FeOOH by controlling the rod length and core-shell structure. The characteristics of α-FeOOH nanorods are controlled by the reaction conditions. In particular, the length of the α-FeOOH rods depends on the concentration of the raw materials, such as the alkali solution. The length of the nanorods is adjusted from 68 nm to 1435 nm. Their yellowness gradually increases, with the highest b* value of 57 based on the International Commission on Illumination (CIE)
Lab system, by controlling the nanorod length. A high quality yellow color is obtained after formation of a silica coating on the α-FeOOH structure. The morphology and the coloration of the nal products are investigated in detail by X-ray diffraction, scanning electron microscopy, UV-vis spectroscopy, and the CIELab color parameter measurements.
- [Korean]
- Coloration Study of Red/Yellow β-FeOOH Nanorod using NH4OH Solution
- Ri Yu, IllJoo Kim, JiYeon Yun, Eun-Young Choi, Jae-Hwan Pee, YooJin Kim
- J Korean Powder Metall Inst. 2016;23(5):343-347. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.343

- 355 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF Fe-based pigments have attracted much interest owing to their eco-friendliness. In particular, the color of nanosized pigments can be tuned by controlling their size and morphology. This study reports on the effect of length on the coloration of β-FeOOH pigments prepared using an NH4OH solution. First, rod-type β-FeOOH is prepared by the hydrolysis of FeCl3·6H2O and NH4OH. When the amount of NH4OH is increased, the length of the rods decreases. Thus, the length of the nanorods can be adjusted from 10 nm to 300 nm. The color of β-FeOOH changes from orangered to yellow depending on the length of β-FeOOH. The color and phase structure of β-FeOOH is characterized by UVvis spectroscopy, CIE Lab color parameter measurements, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and powder X-ray diffraction (XRD).
TOP
 kpmi
kpmi


 First
First Prev
Prev


