Search
- Page Path
- HOME > Search
- [Korean]
- Effects of the Mixing Method and Sintering Temperature on the Characteristics of PZNN-PZT Piezoelectric Ceramic Materials
- So Won Kim, Yong Jeong Jeong, Hee Chul Lee
- J Korean Powder Metall Inst. 2018;25(6):487-493. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.487
- 833 View
- 4 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
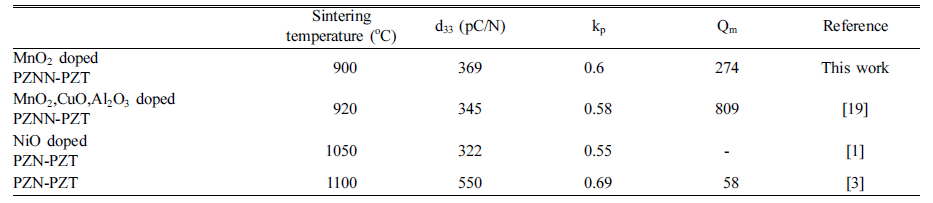
PDF The impact of different mixing methods and sintering temperatures on the microstructure and piezoelectric properties of PZNN-PZT ceramics is investigated. To improve the sinterability and piezoelectric properties of these ceramics, the composition of 0.13Pb((Zn0.8Ni0.2)1/3Nb2/3)O3-0.87Pb(Zr0.5Ti0.5)O3 (PZNN-PZT) containing a Pb-based relaxor component is selected. Two methods are used to create the powder for the PZNN-PZT ceramics. The first involves blending all source powders at once, followed by calcination. The second involves the preferential creation of columbite as a precursor, by reacting NiO with Nb2O5 powder. Subsequently, PZNN-PZT powder can be prepared by mixing the columbite powder, PbO, and other components, followed by an additional calcination step. All the PZNNPZT powder samples in this study show a nearly-pure perovskite phase. High-density PZNN-PZT ceramics can be fabricated using powders prepared by a two-step calcination process, with the addition of 0.3 wt% MnO2 at even relatively low sintering temperatures from 800°C to 1000°C. The grain size of the ceramics at sintering temperatures above 900°C is increased to approximately 3 μm. The optimized PZNN-PZT piezoelectric ceramics show a piezoelectric constant (d33) of 360 pC/N, an electromechanical coupling factor (kp) of 0.61, and a quality factor (Qm) of 275.
-
Citations
Citations to this article as recorded by- An Analysis of Edge Chipping in LiTaO3 Wafer Grinding Using a Scratch Test and FEA Simulation
Haeseong Hwang, Seungho Han, Hyunseop Lee
Lubricants.2023; 11(7): 297. CrossRef - A generalized rule for phase transition generated by additives in piezoelectric ceramics
Jae-Min Cha, Young-Kook Moon, Jung-hwan Kim, Hyun-Ae Cha, Jong-Jin Choi, Byung-Dong Hahn, Seog-Young Yoon, Cheol-Woo Ahn
Materials Today Communications.2023; 37: 107290. CrossRef - Low-Temperature Sintering Properties of Bi2O3 Doped PZT-5H Piezoelectric Ceramics
Wanzi Mao, Qing Xu, Duanping Huang, Huajun Sun, Feng Zhang, Xiaobin Xie
Journal of Electronic Materials.2023; 52(5): 3334. CrossRef - Effect of LiBiO2 on low-temperature sintering of PZT-PZNN ceramics
Sung Cheul Hong, Shi Yeon Kim, Dong-Hun Yeo, Hyo-Soon Shin, Zee Hoon Park, Sahn Nahm
Journal of the Korean Ceramic Society.2022; 59(5): 638. CrossRef - Two-Stage De-binding for Cu Electrode Application to PZT-PZNN Multilayer Actuator
Sung Cheul Hong, Zeehoon Park, Dong-Hun Yeo, Hyo-Soon Shin, Sahn Nahm
Transactions on Electrical and Electronic Materials.2022; 23(4): 348. CrossRef
- An Analysis of Edge Chipping in LiTaO3 Wafer Grinding Using a Scratch Test and FEA Simulation
- [Korean]
- Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
- Woonghee Choi, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(4):287-296. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.287
- 1,579 View
- 31 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
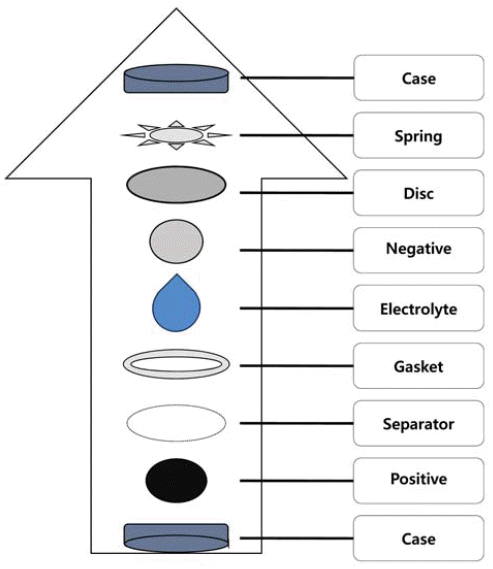
PDF Ni1/3Co1/3Mn1/3(OH)2 powders have been synthesized in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH using NH4OH as a chelating agent. The co-precipitation temperature is varied in the range of 30-80°C. Calcination of the prepared precursors with Li2CO3 for 8 h at 1000°C in air results in Li Ni1/3Co1/3Mn1/3O2 powders. Two kinds of obtained powders have been characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analyzer, and tap density measurements. The co-precipitation temperature does not differentiate the XRD patterns of precursors as well as their final powders. Precursor powders are spherical and dense, consisting of numerous acicular or flaky primary particles. The precursors obtained at 70 and 80°C possess bigger primary particles having more irregular shapes than those at lower temperatures. This is related to the lower tap density measured for the former. The final powders show a similar tendency in terms of primary particle shape and tap density. Electrochemical characterization shows that the initial charge/discharge capacities and cycle life of final powders from the precursors obtained at 70 and 80°C are inferior to those at 50°C. It is concluded that the optimum co-precipitation temperature is around 50°C.
-
Citations
Citations to this article as recorded by- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
Seon Hwa Lee, Ki Young Kwon, Byeong Kil Choi, Hyun Deog Yoo
Journal of Electroanalytical Chemistry.2022; 924: 116828. CrossRef
- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
- [Korean]
- Characteristics of Ni1/3Co1/3Mn1/3(OH)2 Powders Prepared by Co-Precipitation in Air and Nitrogen Atmospheres
- Woonghee Choi, Se-Ryen Park, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(2):136-142. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.136
- 2,342 View
- 41 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
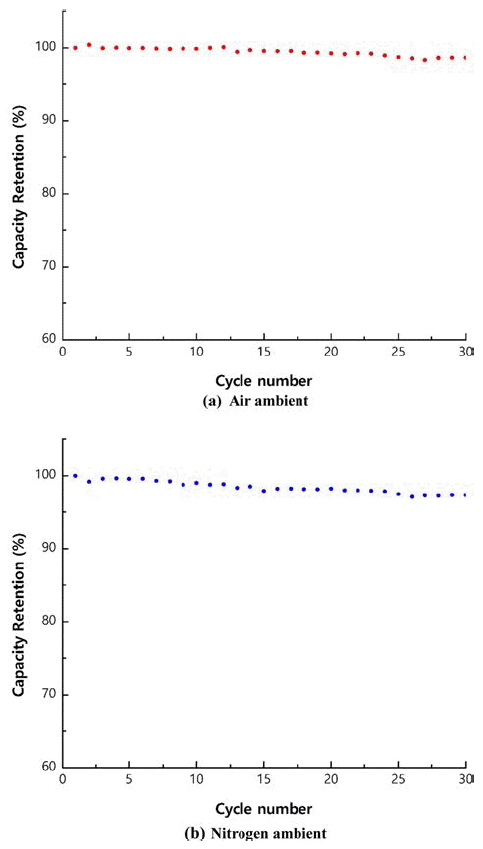
PDF As precursors of cathode materials for lithium ion batteries, Ni1/3Co1/3Mn1/3(OH)2 powders are prepared in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH in the presence of NH4OH in air or nitrogen ambient. Calcination of the precursors with Li2CO3 for 8 h at 1,000°C in air produces dense spherical cathode materials. The precursors and final powders are characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analysis, tap density measurement, and thermal gravimetric analysis. The precursor powders obtained in air or nitrogen ambient show XRD patterns identified as Ni1/3Co1/3Mn1/3(OH)2. Regardless of the atmosphere, the final powders exhibit the XRD patterns of LiNi1/3Co1/3Mn1/3O2 (NCM). The precursor powders obtained in air have larger particle size and lower tap density than those obtained in nitrogen ambient. NCM powders show similar tendencies in terms of particle size and tap density. Electrochemical characterization is performed after fabricating a coin cell using NCM as the cathode and Li metal as the anode. The NCM powders from the precursors obtained in air and those from the precursors obtained in nitrogen have similar initial charge/discharge capacities and cycle life. In conclusion, the powders co-precipitated in air can be utilized as precursor materials, replacing those synthesized in the presence of nitrogen injection, which is the usual industrial practice.
-
Citations
Citations to this article as recorded by- Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
Minjeong Kim, Soonhyun Hong, Heongkwon Jeon, Jahun Koo, Heesang Lee, Gyuseok Choi, Chunjoong Kim
Korean Journal of Materials Research.2022; 32(4): 216. CrossRef - Spherical agglomeration of nickel-manganese-cobalt hydroxide in turbulent Batchelor vortex flow
Xiaotong Sun, Jinsoo Kim, Woo-Sik Kim
Chemical Engineering Journal.2021; 421: 129924. CrossRef - Design strategies for development of nickel-rich ternary lithium-ion battery
Kyu Hwan Choi, Xuyan Liu, Xiaohong Ding, Qiang Li
Ionics.2020; 26(3): 1063. CrossRef - Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 49. CrossRef - Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
Woonghee Choi, Chan Hyoung Kang
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 287. CrossRef
- Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
- Jae-won Lee, Dae Weon Kim, Seong Tae Jang
- J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
- 537 View
- 3 Download
-
 Abstract
Abstract
 PDF
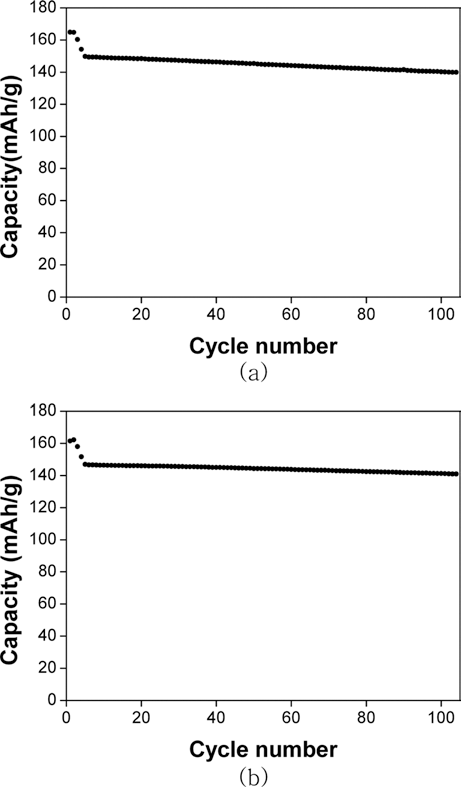
PDF Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
TOP
 KPMI
KPMI


 First
First Prev
Prev


