Search
- Page Path
- HOME > Search
- [Korean]
- Fabrication and Characterisitics of Al2O3-SiC Ceramic Composites for Electrostatic Discharge Safe Components
- Ha-Neul Kim, Hyun-Myung Oh, Young-Jo Park, Jae-Woong Ko, Hyun-Kwuon Lee
- J Korean Powder Metall Inst. 2018;25(2):144-150. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.144
- 517 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF Al2O3-SiC ceramic composites are produced using pressureless sintering, and their plasma resistance, electrical resistance, and mechanical properties are evaluated to confirm their applicability as electrostatic-discharge-safe components for semiconductor devices. Through the addition of Mg and Y nitrate sintering aids, it is confirmed that even if SiC content exceeded 10%, complete densification is possible by pressureless sintering. By the uniform distribution of SiC, the total grain growth is suppressed to about 1 μm; thus an Al2O3-SiC sintered body with a high strength over 600 MPa is obtained. The optimum amount of SiC to satisfy all the desired properties of electrostaticdischarge-safe ceramic components is obtained by finding the correlation between the plasma resistance and the electrical resistivity as a function of SiC amount.
- [Korean]
- Insulating Behavior of Sintered AlN Ceramics Prepared by High-Energy Bead Milling of AlN Powder
- Sung-Soo Ryu, Sung-Min Lee
- J Korean Powder Metall Inst. 2017;24(6):444-449. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.444
- 758 View
- 7 Download
-
 Abstract
Abstract
 PDF
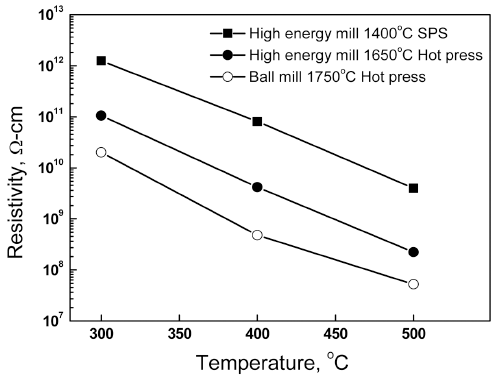
PDF Aluminum nitride (AlN) powder specimens are treated by high-energy bead milling and then sintered at various temperatures. Depending on the solvent and milling time, the oxygen content in the AlN powder varies significantly. When isopropyl alcohol is used, the oxygen content increases with the milling time. In contrast, hexane is very effective at suppressing the oxygen content increase in the AlN powder, although severe particle sedimentation after the milling process is observed in the AlN slurry. With an increase in the milling time, the primary particle size remains nearly constant, but the particle agglomeration is reduced. After spark plasma sintering at 1400°C, the second crystalline phase changes to compounds containing more Al2O3 when the AlN raw material with an increased milling time is used. When the sintering temperature is decreased from 1750°C to 1400°C, the DC resistivity increases by approximately two orders of magnitude, which implies that controlling the sintering temperature is a very effective way to improve the DC resistivity of AlN ceramics.
- [Korean]
- The Effect of SiO2 addition on Oxidation and Electrical Resistance Stability at High-temperature of P/M Fecralloy Compact
- Jin-Woo Park, Jin-Uk Ok, Woo-young Jung, Dong-kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2017;24(4):292-297. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.292
- 617 View
- 2 Download
-
 Abstract
Abstract
 PDF
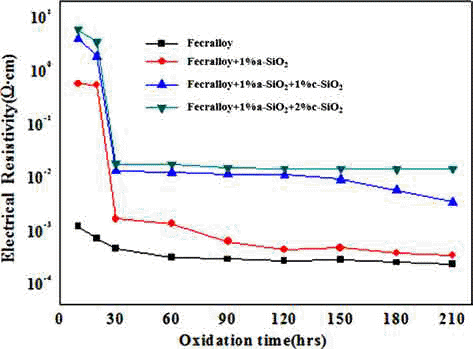
PDF A metallic oxide layer of a heat-resistant element contributes to the high-temperature oxidation resistance by delaying the oxidation and has a positive effect on the increase in electrical resistivity. In this study, green compacts of Fecralloy powder mixed with amorphous and crystalline silica are oxidized at 950°C for up to 210 h in order to evaluate the effect of metal oxide on the oxidation and electrical resistivity. The weight change ratio increases as per a parabolic law, and the increase is larger than that observed for Fecralloy owing to the formation of Fe-Si, Fe-Cr composite oxide, and Al2O3 upon the addition of Si oxide. Si oxides promote the formation of Al2O3 and Cr oxide at the grain boundary, and obstruct neck formation and the growth of Fecralloy particles to ensure stable electrical resistivity.
TOP
 KPMI
KPMI

 First
First Prev
Prev


