Search
- Page Path
- HOME > Search
- [Korean]
- A Study on the Preparation and Growth Mechanism of Titanium Dioxide using Organic-Inorganic Hybrid Titanium Complex
- Yubin Kang, Jin-Ju Choi, Nam Hun Kwon, Dae-Guen Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(6):487-492. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.487
- 1,152 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
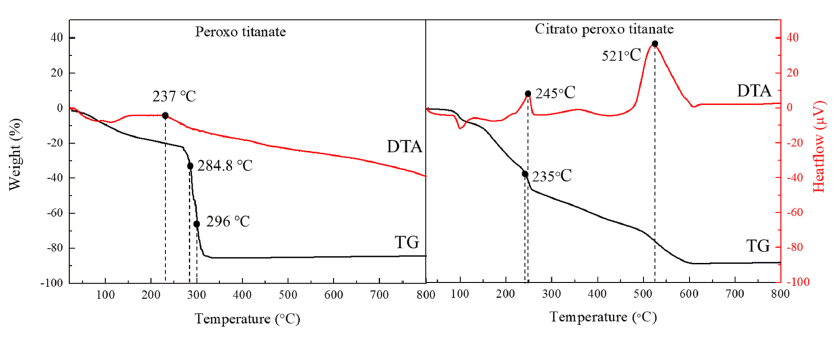
PDF Titanium dioxide (TiO2) is a typical inorganic material that has an excellent photocatalytic property and a high refractive index. It is used in water/air purifiers, solar cells, white pigments, refractory materials, semiconductors, etc.; its demand is continuously increasing. In this study, anatase and rutile phase titanium dioxide is prepared using hydroxyl and carboxyl; the titanium complex and its mechanism are investigated. As a result of analyzing the phase transition characteristics by a heat treatment temperature using a titanium complex having a hydroxyl group and a carboxyl group, it is confirmed that the material properties were different from each other and that the anatase and rutile phase contents can be controlled. The titanium complexes prepared in this study show different characteristics from the titania-formation temperatures of the known anatase and rutile phases. It is inferred that this is due to the change of electrostatic adsorption behavior due to the complexing function of the oxygen sharing point, which crystals of the TiO6 structure share.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
- Synthesis of TiO2 Nanowires by Thermal Oxidation of Titanium Alloy Powder
- Yoo-Young Kim, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2018;25(1):48-53. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.48

- 736 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF One-dimensional rutile TiO2 is an important inorganic compound with applicability in sensors, solar cells, and Li-based batteries. However, conventional synthesis methods for TiO2 nanowires are complicated and entail risks of environmental contamination. In this work, we report the growth of TiO2 nanowires on a Ti alloy powder (Ti-6wt%Al-4wt%V, Ti64) using simple thermal oxidation under a limited supply of O2. The optimum condition for TiO2 nanowire synthesis is studied for variables including temperature, time, and pressure. TiO2 nanowires of ~5 μm in length and 100 nm in thickness are richly synthesized under the optimum condition with single-crystalline rutile phases. The formation of TiO2 nanowires is greatly influenced by synthesis temperature and pressure. The synthesized TiO2 nanowires are characterized using field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and high-resolution transmission electron microscopy (HR-TEM).
- [Korean]
- Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
- J Korean Powder Metall Inst. 2016;23(5):353-357. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.353
- 605 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
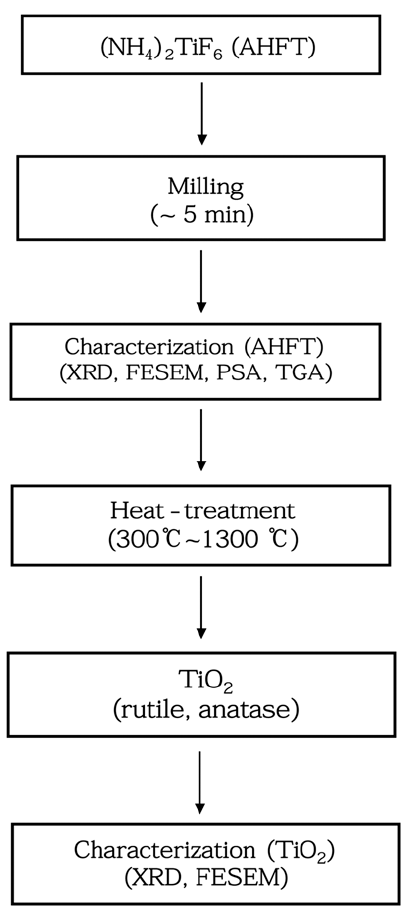
PDF In this study, TiO2 powders are synthesized from ammonium hexafluoride titanate (AHFT, (NH4)2TiF6) as a precursor by heat treatment. First, we evaluate the physical properties of AHFT using X-ray diffraction (XRD), particle size analysis (PSA), thermogravimetric analysis (TGA), and field-emission scanning electron microscopy (FESEM). Then, to prepare the TiO2 powders, is heat-treated at 300-1300°C for 1 h. The ratio of anatase to rutile phase in TiO2 is estimated by XRD. The anatase phase forms at 500°C and phase transformation to the rutile phase occurs at 1200°C. Increase in the particle size is observed upon increasing the reaction temperature, and the phase ratio of the rutile phase is determined from a comparison with the calculated XRD data. Thus, we show that anatase and rutile TiO2 powders could be synthesized using AHFT as a raw material, and the obtained data are utilized for developing a new process for producing high-quality TiO2 powder.
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


