Search
- Page Path
- HOME > Search
- [English]
- Design of Conductive Inks Containing Carbon Black and Silver Nanowires for Patternable Screen-Printing on Fabrics
- Seokhwan Kim, Geumseong Lee, Jinwoo Park, Dahye Shin, Ki-Il Park, Kyoung Jin Jung, Yuho Min
- J Powder Mater. 2024;31(6):500-507. Published online December 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00409
- 1,989 View
- 58 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
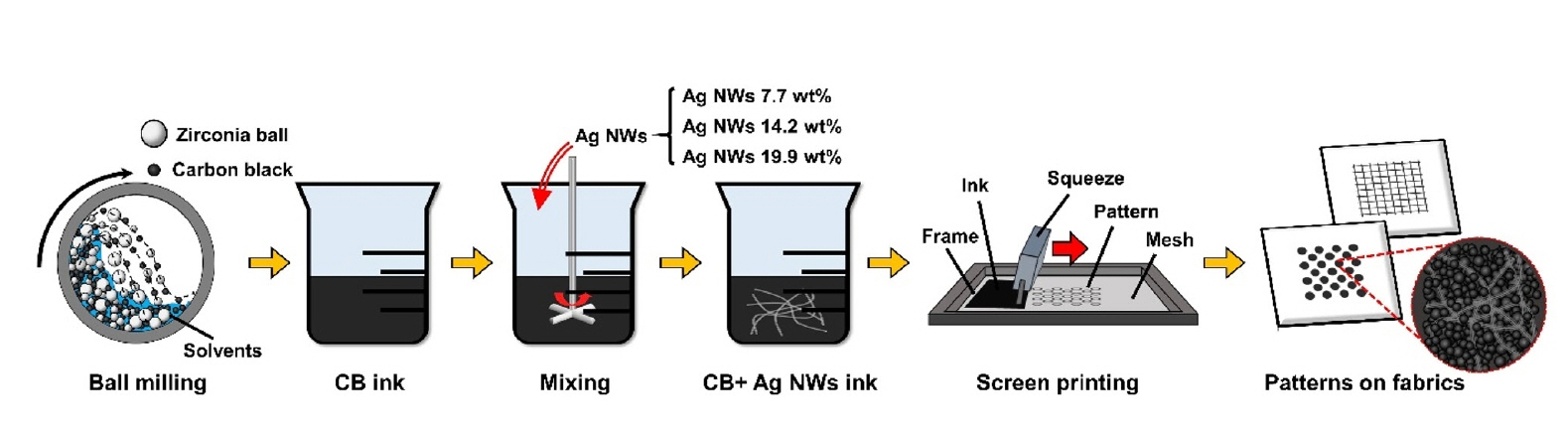
PDF - This study developed conductive inks composed of carbon black (CB) and silver nanowires (Ag NWs) for cost-effective screen-printing on fabrics. The Ag NW density within the CB matrix was precisely controlled, achieving tunable electrical conductivity with minimal Ag NW usage. The resulting inks were successfully patterned into shapes such as square grids and circles on textile surfaces, demonstrating excellent conductivity and fidelity. Adding 19.9 wt% Ag NWs reduced sheet resistance by ~92% compared to CB-only inks, highlighting the effectiveness and potential of this hybrid approach for cost-effective, high-performance textile-based electronics. The one-dimensional morphology of Ag NWs facilitated the formation of conductive percolation networks, creating efficient electron pathways within the CB matrix even at low loadings. This work advances the field of CB-based conductive inks and provides a scalable and practical method for producing functional, patterned electronic textiles.
-
Citations
Citations to this article as recorded by- Multifunctional Screen-Printed Conductive Inks: Design Principles, Performance Challenges, and Application Horizons
Nahid Islam, Manisha Das, Bashir Ahmed Johan, Syed Shaheen Shah, Atif Saeed Alzahrani, Md. Abdul Aziz
ACS Applied Electronic Materials.2025; 7(16): 7503. CrossRef
- Multifunctional Screen-Printed Conductive Inks: Design Principles, Performance Challenges, and Application Horizons
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 884 View
- 4 Download
-
 Abstract
Abstract
 PDF
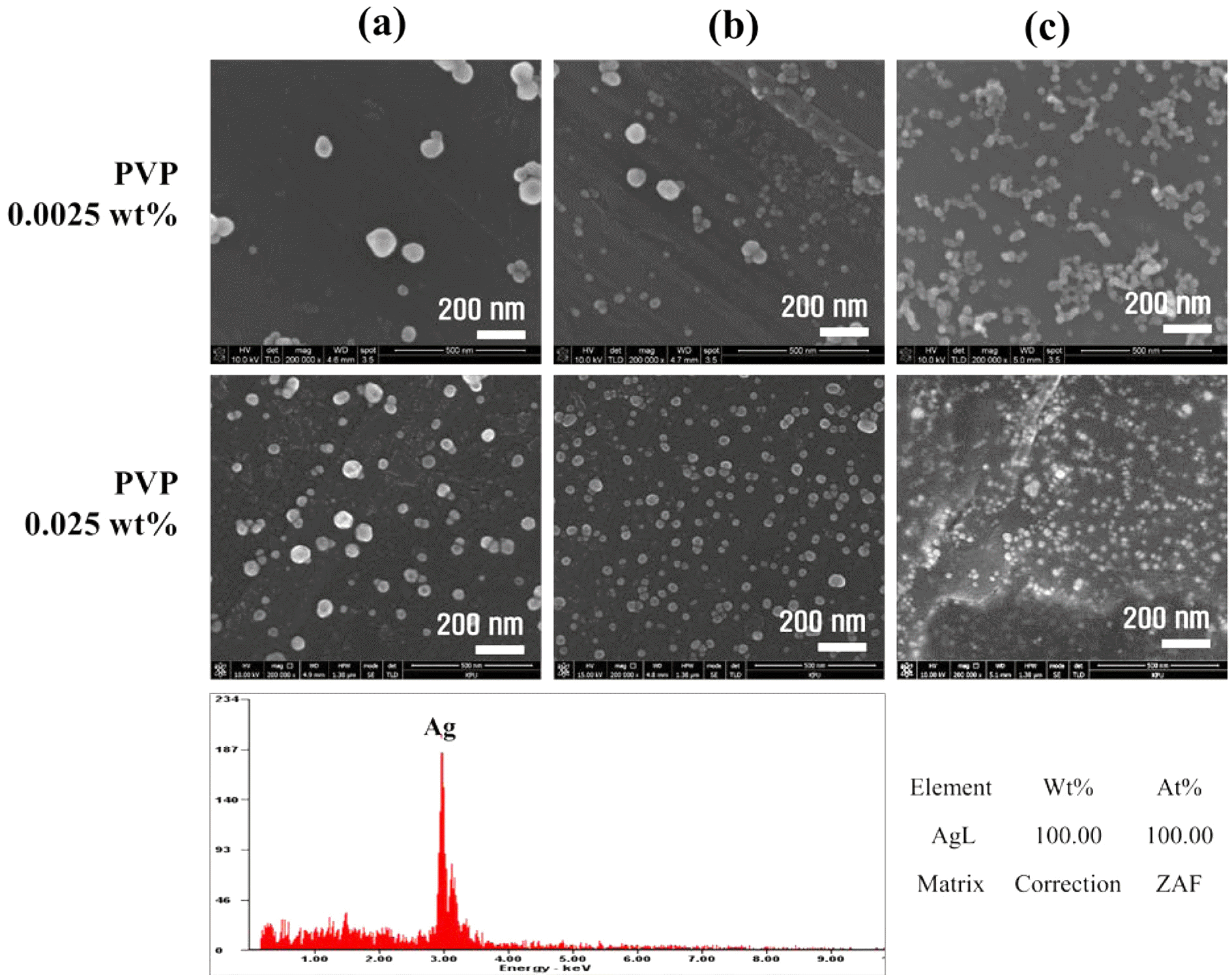
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- Filling and Wiping Properties of Silver Nano Paste in Trench Layer of Metal Mesh Type Transparent Conducting Electrode Films for Touch Screen Panel Application
- Gi-Dong Kim, Hyun-Min Nam, Sangsun Yang, Lee-Soon Park, Su-Yong Nam
- J Korean Powder Metall Inst. 2017;24(6):464-471. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.464

- 1,150 View
- 8 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
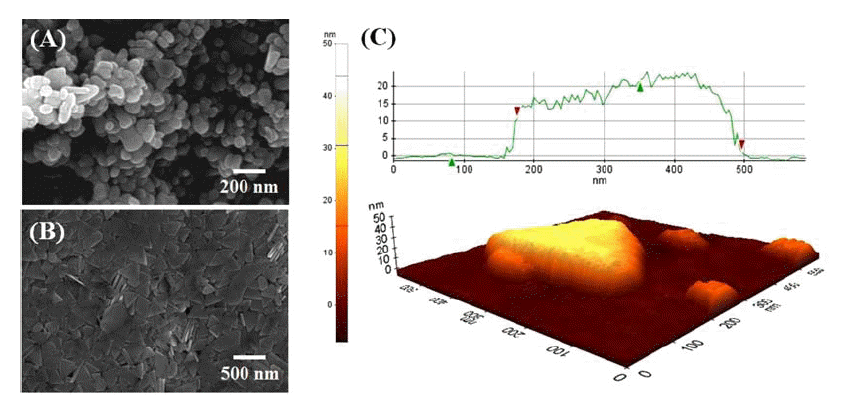
PDF A metal mesh TCE film is fabricated using a series of processes such as UV imprinting of a transparent trench pattern (with a width of 2-5 μm) onto a PET film, filling it with silver paste, wiping of the surface, and heatcuring the silver paste. In this work nanosized (40-50 nm) silver particles are synthesized and mixed with submicron (250-300 nm)-sized silver particles to prepare silver paste for the fabrication of metal mesh-type TCE films. The filling of these silver pastes into the patterned trench layer is examined using a specially designed filling machine and the rheological testing of the silver pastes. The wiping of the trench layer surface to remove any residual silver paste or particles is tested with various mixture solvents, and ethyl cellosolve acetate (ECA):DI water = 90:10 wt% is found to give the best result. The silver paste with 40-50 nm Ag:250-300 nm Ag in a 10:90 wt% mixture gives the highest electrical conductance. The metal mesh TCE film obtained with this silver paste in an optimized process exhibits a light transmittance of 90.4% and haze at 1.2%, which is suitable for TSP application.
-
Citations
Citations to this article as recorded by- Silver and epoxy binder-based printed electrodes and the effect of silver nanoparticles on stretchability
Suk Hun Hyun, Se-Hoon Park, Sung-Hoon Choa, Hyun Jin Nam, Heejoon Ahn
Journal of Materials Science: Materials in Electronics.2019; 30(19): 17591. CrossRef - Electro-mechanical Properties of Stretchable Ag Paste by the Difference of Ag Particles
Sun-Young Kang, Min-Young Park, Dong-Young Jang
Journal of the Korean Society of Manufacturing Technology Engineers.2019; 28(3): 188. CrossRef
- Silver and epoxy binder-based printed electrodes and the effect of silver nanoparticles on stretchability
- [Korean]
- Investigation on Microstructure and Electrical Properties of Silver Conductive Features Using a Powder Composed of Silver nanoparticles and Nanoplatelets
- Yong-Sung Goo, Yong-Ho Choa, Young Hwangbo, Young-In Lee
- J Korean Powder Metall Inst. 2016;23(5):358-363. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.358
- 566 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF Noncontact direct-printed conductive silver patterns with an enhanced electrical resistivity are fabricated using a silver ink with a mixture of silver nanoparticles and nanoplates. The microstructure and electrical resistivity of the silver pattern are systematically investigated as a function of the mixing ratio of the nanoparticles and nanoplates. The pattern, which is fabricated using a mixture with a mixing ratio of 3(nanoparticles):7(nanoplates) and sintered at 200°C shows a highly dense and well-sintered microstructure and has a resistivity of 7.60 μΩ·cm. This originates a mutual synergistic effect through a combination of the sinterability of the nanoparticles and the packing ability of the nanoplates. This is a conductive material that can be used to fabricate noncontact direct-printed conductive patterns with excellent electrical conductivity for various flexible electronics applications, including solar cells, displays, RFIDs, and sensors.
TOP
 KPMI
KPMI


 First
First Prev
Prev


