Search
- Page Path
- HOME > Search
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 816 View
- 4 Download
-
 Abstract
Abstract
 PDF
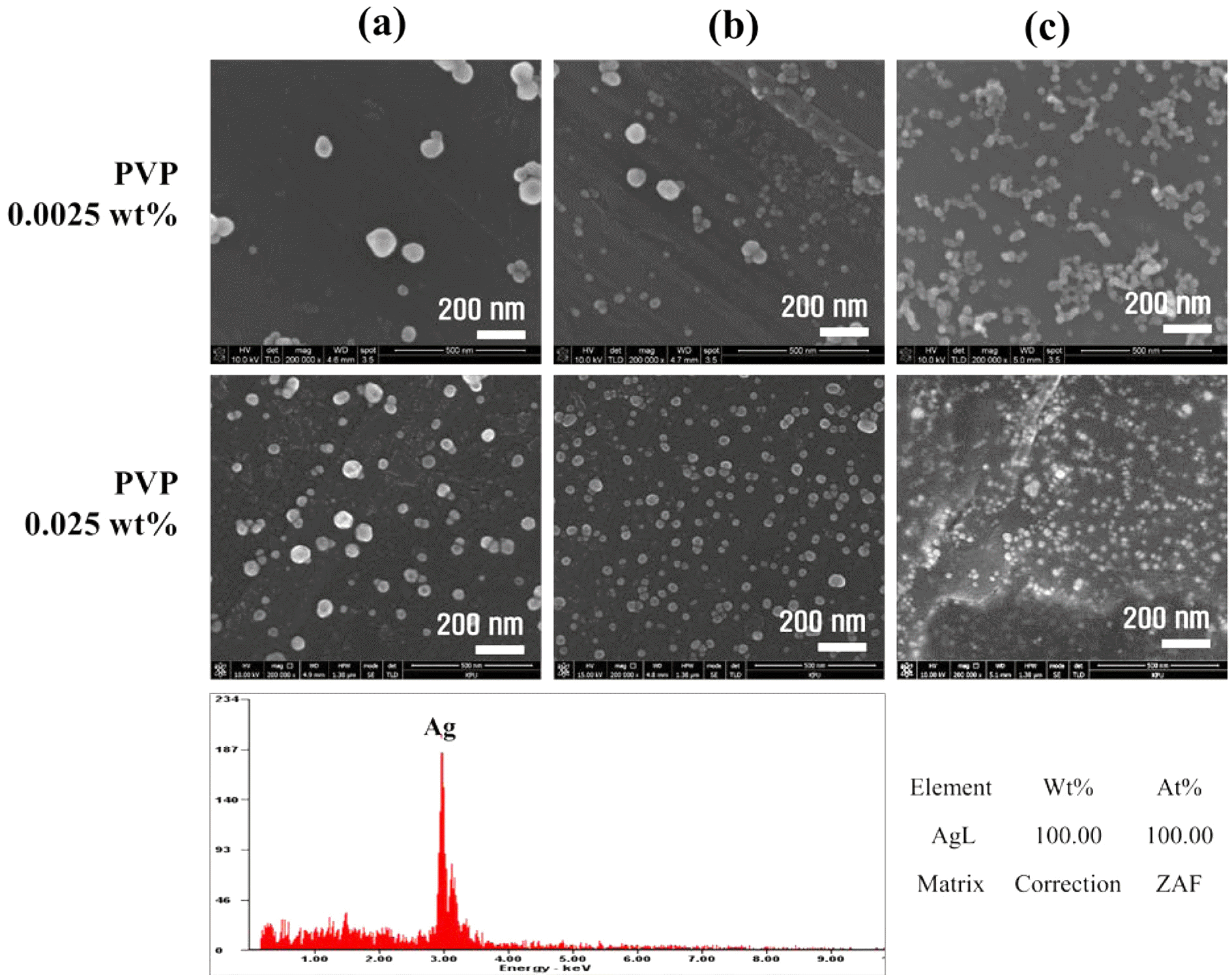
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- The Preparation of Dye-Sensitized Solar Cell Paste Used the Peroxo Titanium Complex and Characteristics by Annealing Temperature
- Hyunsu Park, Soyeong Joo, Joon-Phil Choi, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2015;22(6):396-402. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.396
- 888 View
- 5 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
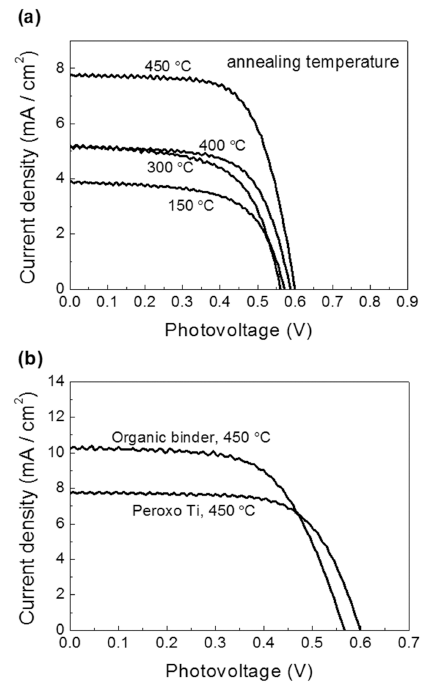
PDF The organic binder-free paste for dye-sensitized solar cell (DSSC) has been investigated using peroxo titanium complex. The crystal structure of TiO2 nanoparticles, morphology of TiO2 film and electrical properties are analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Electrochemical Impedance Spectra (EIS), and solar simulator. The synthesized TiO2 nanopowders by the peroxo titanium complex at 150, 300, 400°C, and 450°C have anatase phase and average crystal sizes are calculated to be 4.2, 13.7, 16.9, and 20.9 nm, respectively. The DSSC prepared by the peroxo titanium complex binder have higher Voc and lower Jsc values than that of the organic binder. It can be attributed to improvement of sintering properties of TCO/TiO2 and TiO2/TiO2 interface and to formation of agglomerate by the nanoparticles. As a result, we have investigated the organic binder-free paste and 3.178% conversion efficiency of the DSSC at 450°C.
-
Citations
Citations to this article as recorded by- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
Won Jin Kim, Jinho Lee, Ryun Na Kim, Donghee Lee, Woo-Byoung Kim
Korean Journal of Materials Research.2024; 34(3): 152. CrossRef - Development of eco-friendly thin film manufacturing process using poeroxo titanium complex solution and potential for resistive random access memory
Jinho Lee, Ryun Na Kim, Kee-Ryung Park, Woo-Byoung Kim
Applied Surface Science.2021; 562: 150170. CrossRef - Preparation of ultra-thin TiO2 shell by peroxo titanium complex (PTC) solution-based green surface modification, and photocatalytic activity of homo-core/shell TiO2
Jinho Lee, Jiyong Hwang, Hyunsu Park, Tohru Sekino, Woo-Byoung Kim
Applied Surface Science.2021; 540: 148399. CrossRef - Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion
Hyunsu Park, Tomoyo Goto, Sunghun Cho, Soo Wohn Lee, Masato Kakihana, Tohru Sekino
Nanomaterials.2020; 10(7): 1331. CrossRef - Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(5): 353. CrossRef
- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
TOP
 KPMI
KPMI


 First
First Prev
Prev


