Search
- Page Path
- HOME > Search
Research Articles
- [Korean]
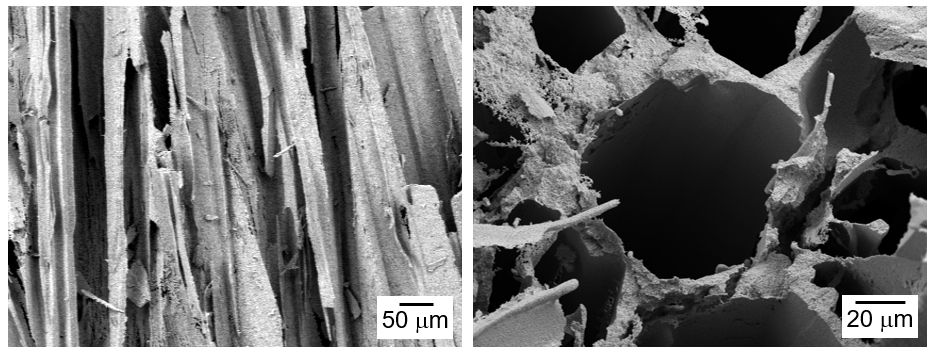
- Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
- Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
- J Powder Mater. 2025;32(6):466-471. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00437
- 607 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF - The influence of process conditions on the microstructure of porous W-Cu, fabricated by freeze casting using tert-butyl alcohol as the freezing agent, was investigated. The slurries containing 10 vol% of WO3-CuO powder were prepared by milling with a small amount of citric acid and polyethylene glycol as dispersants. The slurries with dispersion stability were frozen in a mold with the lower part cooled to -25°C, followed by sublimation in a vacuum to remove the freezing agent. The sintered W-1 vol% Cu in a hydrogen atmosphere exhibited aligned pores with the size of 50 μm, which were generated by sublimation of directionally solidified tert-butyl alcohol crystals. In the cross-section of the specimen, hexagonal pores corresponding to the crystal structure of tert-butyl alcohol was observed. Microstructure analysis of the struts revealed that Cu was distributed non-uniformly due to the mutual insolubility and low wettability of the W-Cu system.
- [Korean]
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(4):324-328. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00199

- 999 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - This study analyzed the influence of ball size and process control agents on the refinement and dehydrogenation behavior of TiH2 powder. Powders milled using ZrO2 balls with diameters of 0.1 mm, 0.3 mm, and 0.3+0.5+1 mm exhibited a bimodal particle size distribution, of which the first mode had the smallest size of 0.23 μm for the 0.3 mm balls. Using ethanol and/or stearic acid as process control agents was effective in particle refinement. Thermogravimetric analysis showed that dehydrogenation of the milled powder started at a relatively low temperature compared to the raw powder, which is interpreted to have resulted from a decrease in particle size and an increase in defects. The dehydrogenation kinetics of the TiH2 powder were evaluated by the magnitude of peak shift with heating rates using thermogravimetric analysis. The activation energy of the dehydrogenation reaction, calculated from the slope of the Kissinger plot, was measured to be 228.6 kJ/mol for the raw powder and 194.5 kJ/mol for the milled powder. TEM analysis revealed that both the milled and dehydrogenated powders showed an angular shape with a size of about 200 nm.
- [Korean]
- Effect of Ball Milling Conditions on the Microstructure and Dehydrogenation Behavior of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(2):132-136. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00001

- 2,050 View
- 37 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - This study investigated the effects of revolution speed and ball size in planetary milling on the microstructure and dehydrogenation behavior of TiH2 powder. The particle size analysis showed that the large particles present in the raw powder were effectively refined as the revolution speed increased, and when milled at 500 rpm, the median particle size was 1.47 m. Milling with a mixture of balls of two or three sizes was more effective in refining the raw powder than milling with balls of a single size. A mixture of 3-mm and 5-mm-diameter balls was the optimal condition for particle refinement, and the measured median particle size was 0.71 m. The dependence of particle size on revolution speed and ball size was explained by changes in input energy and the number of contact points of the balls. In the milled powder, the endothermic peak measured using differential thermal analysis was observed at a relatively low temperature. This finding was interpreted as the activation of a dehydrogenation reaction, mainly due to the increase in the specific surface area and the concentration of lattice defects.
-
Citations
Citations to this article as recorded by- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
Journal of Powder Materials.2024; 31(4): 324. CrossRef
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
TOP
 KPMI
KPMI


 First
First Prev
Prev


