Search
- Page Path
- HOME > Search
- [Korean]
- Extractive Metallurgy and Recycling of Cobalt
- Ho-Sang Sohn
- J Powder Mater. 2022;29(3):252-261. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.252
- 1,203 View
- 16 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
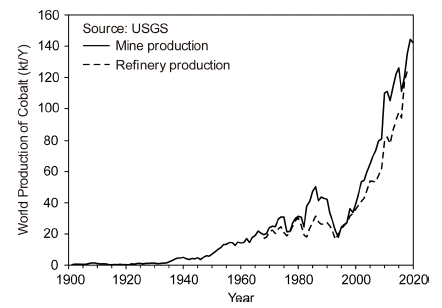
PDF Cobalt is a vital metal in the modern society because of its applications in lithium-ion batteries, super alloys, hard metals, and catalysts. Further, cobalt is a representative rare metal and is the 30th most abundant element in the Earth’s crust. This study reviews the current status of cobalt extraction and recycling processes, along with the trends in its production amount and use. Although cobalt occurs in a wide range of minerals, such as oxides and sulfides of copper and nickel ores, the amounts of cobalt in the minerals are too low to be extracted economically. The Democratic Republic of Congo (DRC) leads cobalt mining, and accounts for 68.9 % of the global cobalt reserves (142,000 tons in 2020). Cobalt is mainly extracted from copper–cobalt and nickel–cobalt concentrates and is occasionally extracted directly from the ore itself by hydro-, pyro-, and electro-metallurgical processes. These smelting methods are essential for developing new recycling processes to extract cobalt from secondary resources. Cobalt is mainly recycled from lithium-ion batteries, spent catalysts, and cobalt alloys. The recycling methods for cobalt also depend on the type of secondary cobalt resource. Major recycling methods from secondary resources are applied in pyro- and hydrometallurgical processes.
-
Citations
Citations to this article as recorded by- Reduction Behavior of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries by CO Gas
Sang-Yeop Lee, Jae-Ho Hwang, So-Yeong Lee, Ho-Sang Sohn
Korean Journal of Metals and Materials.2025; 63(10): 820. CrossRef - Recovering cobalt from cobalt oxide ore using suspension roasting and magnetic separation technique
Xinlei Wei, Yongsheng Sun, Yanjun Li, Peng Gao
Journal of Materials Research and Technology.2023; 27: 3005. CrossRef
- Reduction Behavior of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries by CO Gas
- [Korean]
- Recovery of Tungsten from WC/Co Hardmetal Sludge by Alkaline Leaching Hydrometallurgy Process
- Gil-Geun Lee, Ji-Eun Kwon
- J Korean Powder Metall Inst. 2016;23(5):372-378. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.372
- 839 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF This study focuses on the development of an alkaline leaching hydrometallurgy process for the recovery of tungsten from WC/Co hardmetal sludge, and an examination of the effect of the process parameters on tungsten recovery. The alkaline leaching hydrometallurgy process has four stages, i.e., oxidation of the sludge, leaching of tungsten by NaOH, refinement of the leaching solution, and precipitation of tungsten. The WC/Co hardmetal sludge oxide consists of WO3 and CoWO4. The leaching of tungsten is most affected by the leaching temperature, followed by the NaOH concentration and the leaching time. About 99% of tungsten in the WC/Co hardmetal sludge is leached at temperatures above 90°C and a NaOH concentration above 15%. For refinement of the leaching solution, pH control of the solution using HCl is more effective than the addition of Na2S·9H2O. The tungsten is precipitated as high-purity H2WO4·H2O by pH control using HCl. With decreasing pH of the solution, the tungsten recovery rate increases and then decrease. About 93% of tungsten in the WC/Co hardmetal sludge is recovered by the alkaline leaching hydrometallurgy process.
-
Citations
Citations to this article as recorded by- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
Min Soo Park, Jong-Min Gwak, Kyeong-mi Jang, Gook-Hyun Ha
Powder Metallurgy.2023; 66(5): 688. CrossRef
- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
TOP
 KPMI
KPMI


 First
First Prev
Prev


