Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 28(2); 2021 > Article
-
Article
- Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process
- Sangmin Parka,b, Sun-Woo Nama, Sang-Hoon Leea, Myung-Suk Songa, Taek-Soo Kima,b,*
-
Journal of Korean Powder Metallurgy Institute 2021;28(2):91-96.
DOI: https://doi.org/10.4150/KPMI.2021.28.2.91
Published online: March 31, 2021
a Research Institute of Advanced Manufacturing Technology, Incheon 21999, Republic of Korea
b Industrial Technology, Korea University of Science and Technology, Daejeon 34113, Republic of Korea
- *Corresponding Author: Dr. Taek-Soo Kim, TEL: +82-32-458-5118, FAX: +82-31-458-5112, E-mail: tskim@kitech.re.kr
- - 박상민·남선우·이상훈: 학생, 송명석: 포스트닥터, 김택수: 수석연구원
• Received: February 10, 2021 • Revised: March 2, 2021 • Accepted: March 2, 2021
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,763 Views
- 21 Download
- 6 Crossref
Abstract
- Rare earth magnets with excellent magnetic properties are indispensable in the electric device, wind turbine, and e-mobility industries. The demand for the development of eco-friendly recycling techniques has increased to realize sustainable green technology, and the supply of rare earth resources, which are critical for the production of permanent magnets, are limited. Liquid metal extraction (LME), which is a type of pyrometallurgical recycling, is known to selectively extract the metal forms of rare earth elements. Although several studies have been carried out on the formation of intermetallic compounds and oxides, the effect of oxide formation on the extraction efficiency in the LME process remains unknown. In this study, microstructural and phase analyses are conducted to confirm the oxidation behavior of magnets pulverized by a jaw crusher. The LME process is performed with pulverized scrap, and extraction percentages are calculated to confirm the effect of the oxide phases on the extraction of Dy during the reaction. During the LME p rocess, Nd i s completely e xtracted a fter 6 h, w hile D y remains as D y2Fe17 and Dy-oxide. Because the decomposition rate of Dy2Fe17 is faster than the reduction rate of Dy-oxide, the importance of controlling Dy-oxide on Dy extraction is confirmed.
- The permanent magnet is becoming eminent alternative for generating energy instead of fossil fuel since it can convert electrical signal to energy for operating devices. Therefore, the permanent magnet is becoming increasingly crucial as the world shifts its focus towards greener technology. Among them, Nd-Fe-B permanent magnet has outstanding magnetic properties as compared to the other permanent magnets such as ferrite and alnico magnet [1]. Although, Nd based permanent magnets has superior magnetic properties, it can’t retain its properties in high operating temperature. Therefore, Dy is added to improve coercivity in order to prevent losing magnetic properties at elevated temperatures [2, 3]. However, most countries are confronted with a supply risk of these REEs since their resources are observed in a limited number of countries compared to increasing the demands. Therefore, many researchers are now focusing on recycling processes in order to circulate the available REEs resources [4-10].
- Hydro-metallurgical recycling method such as solvent extraction which is similar to extract REEs from ore is selected for high extraction efficiency and high purity of its byproduct. However, chemicals are used in hydrometallurgical recycling even if usage of chemicals is less than extraction from ore, severe environmental damages posed from chemical wastes can be caused [4-7]. Therefore, research on pyro-metallurgical method is becoming an attractive alternatives instead of hydro-metallurgical method [8-10].
- Liquid metal extraction (LME) which is one of the pyro-metallurgical recycling techniques have been carried actively on rare earths based permanent magnet due to its simple process stages and metal formed byproduct. Nd can be extracted within 6 hours of extraction while extraction rate of Dy is relatively slow. The cause is assumed to be the formation of intermetallic compounds and oxide due to the high reactivity of Dy with Fe and oxygen during reaction acting as hindrance for the Dy extraction [11]. Research on optimization of process parameters is also observed actively and these studies are focused on how much Dy2Fe17 and Dy-oxide changes during extraction process [12]. However, There is a lack of systematic investigation on the oxide formation in the (Nd, Dy)-Fe-B magnets.
- The aim for this study is the investigation of the effect of oxidation behavior of (Nd,Dy)-Fe-B magnet on Dy extraction by LME. This study shows the oxidation behavior of (Nd, Dy)-Fe-B magnet and its effect on extraction behavior of Dy to Mg using pulverized scrap which has different oxygen contents by size under microstructural aspect and phase analysis.
1. Introduction
- 39UH grade magnets supplied by JAHWA electronic Co. Ltd, having a Dy content of about 3.5 wt.%, were used as raw material and their composition is shown in Table 1. The magnet was pulverized into scrap of 200- 300 microns, 300-710 microns, 710-1000 microns and 1000-2000 microns sizes using a jaw crusher. Oxygen contents by size of pulverized scrap was analyzed by Nitrogen-Oxygen-Hydrogen (NOH) analyzer. In order to confirm oxidation of the pulverized scrap, Microstructure was analyzed by line scanning of scanning electron microscope (FE-SEM, Model JSM-100F) equipped with energy dispersive X-ray analysis (EDS) and phases in scrap were analyzed by X-ray diffraction (XRD, Model D/Max 2500PC).
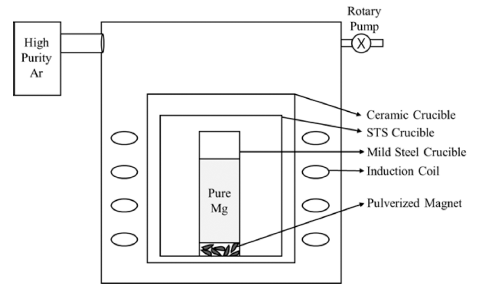
- After analysis for oxidation of the magnet, LME was processed with 99% pure Mg, supplied by JC company which does not react with Fe and B during reaction. Reaction experiment was carried out in a high frequency induction furnace (Model DTIH-0020VMF, Korea) which helps circulate liquid Mg with optimized Mg ratio (WMagnet:WMg = 1:10) and temperature (900°C)[12]. Schematics of LME experiment is indicated in Fig. 1. Changes of volume fractions of each phase were analyzed by image analyzer (Program, ImageJ) using the microstructures of the reacted sample obtained by FESEM. Image analyzer measures phase fractions using differences of brightness of each phase. Extraction percentages was calculated by the equation [13] using results by X-ray Fluorescence Spectrometry (XRF, Model ARL PERFORM’X). Finally, effect of oxidation behavior of (Nd,Dy)-Fe-B magnet on Dy extraction by LME can be confirmed by these analysis.
2. Experimental
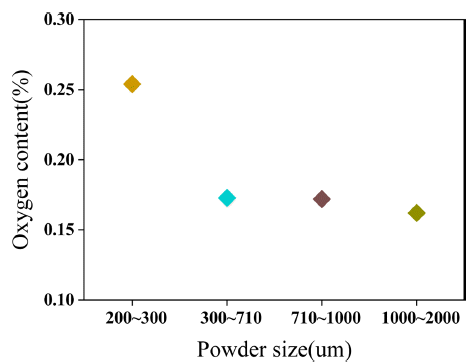
- Magnet can be easily oxidized during pulverization process using jaw crusher since REEs in magnet have strong reactivity with oxygen at grinding environment. Variation of oxygen contents by scrap size is shown in Fig. 2. 200-300 microns sized scrap had the highest oxygen contents about 2612 ppm and 1000-2000 microns sized scrap had the lowest oxygen contents about 1621 ppm. Difference of oxygen contents between 200-300 microns sized scrap and 1000-2000 microns sized scrap was about 991 ppm. Difference of oxidation by size of pulverized scrap was generated by volumetric surface for reaction with oxygen.
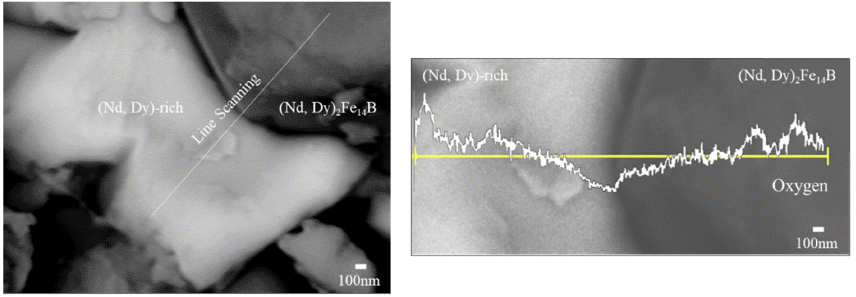
- In the previous research, the oxidation behavior of Nd based permanent magnet without Dy was observed. The Nd-rich and Nd2Fe14B phases transformed to form Ndoxide and NdFeO3 [14]. Microstructural analysis of 200- 300 microns sized scrap which is highly oxidized compared to the other scrap sizes was observed to confirm oxidation behavior of Dy added magnet. The results of microstructural analysis are shown in Fig. 3. In the case of Dy added magnet, Dy coexists with Nd as (Nd, Dy)2Fe14B phases located in grain and (Nd, Dy)-rich phases located in grain boundary hence Dy was oxidized with Nd in the same phase. Oxygen concentration was highly detected in specific area of (Nd, Dy)-rich phase and (Nd, Dy)2Fe14B phase regions. Oxygen was highly detected in the surface of (Nd, Dy)-rich and (Nd, Dy)2Fe14B. Therefore, Nucleation of a new phase containing oxygen was indicated by microstructural analysis.
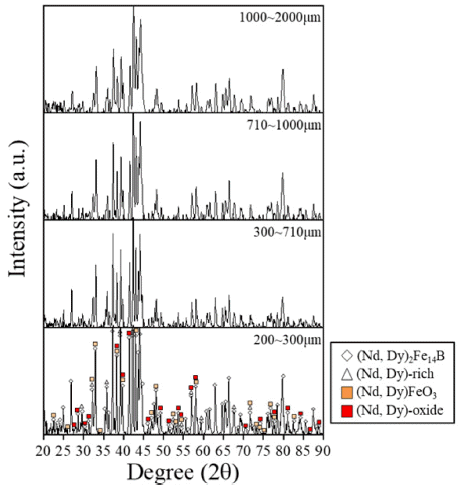
- In order to confirm what oxide phases were formed in the magnet, the XRD analysis was carried out by the size of pulverized scrap. Although, oxide phases were not apparently revealed due to overlapping with (Nd, Dy)2 Fe14B, main peaks of (Nd, Dy)-oxide and (Nd, Dy)FeO3 were matched as shown in Fig. 4. (Nd, Dy)-oxide and (Nd, Dy)FeO3 peaks were shifted about 0.34 degrees by Dy addition than Nd-oxide and NdFeO3 due to difference of atomic radius between Nd and Dy. Oxide phases were detected in whole range of size of pulverized scrap. Therefore, Generation of the oxide phases during the oxidation of the magnet was confirmed.
- Liquid metal extraction using Mg was conducted to confirm oxidation effect during extraction behavior under optimized Mg ratio and process temperature [12] with variation of process time from 6 hours to 48 hours and size of pulverized scrap. Microstructures at 48 hours by size of pulverized scrap are shown in Fig. 5. Nd was not detected since it is completely extracted by the Mg [15] while Dy was detected in the form of Dy2Fe17 in grain and Dy-oxide in grain boundary. Previous researches have shown that the Dy2Fe17 is generated from (Nd, Dy)2Fe14B due to reactivity between Dy and Fe during extraction process [11, 12]. Any phase including oxygen was not detected except Dy-oxide. Mixing enthalpy between Nd and Mg is 1 value more negative than Dy and Mg. In addition, Nd has less affinity with oxygen than Dy as indicated in ellingham diagram [12]. Therefore, Nd in (Nd, Dy)-oxide and (Nd, Dy)FeO3 is reduced and extracted to Mg since Nd has relatively strong affinity with Mg and relatively weak affinity with oxygen compared to Dy. Therefore, transformation from (Nd, Dy)-oxide and (Nd, Dy)FeO3 phases to Dy-oxide during extraction process was confirmed from the results of this study.
- In order to observe effect of oxidation of magnet on extraction of Dy, the volume fractions of Dy2Fe17 and Dy-oxide were compared with increasing reaction time and pulverized scrap size in Fig. 6. Measured volume fraction is visualized in Fig. 6 by image analysis using results in Fig. 5. 200-300 microns sized scrap had the highest volume fraction of Dy-oxide about 1.49% at 6 hours and 1000-2000 microns sized scrap had the least volume fraction of Dy-oxide about 1.15% at 6 hours as shown in Fig. 6(a). Formation of phases including oxygen was proceeded as size of pulverized scrap decreases. Therefore, 200-300 microns sized scrap was highly oxidized compared to the other scrap so the highest amount of transformation of oxide phases to Dy-oxide had taken place. Phases including oxygen were less frequently observed as size of pulverized scrap increases hence lesser amount of transformation of oxide phases to Dyoxide was observed.
- Even as the reduction is proceeded with process time, Dy-oxide contents in 200-300 microns size of pulverized scrap remained 0.1-0.2% higher than the other scrap sizes until 48 hours. The remained Dy-oxide in other sized scrap followed a trend of oxygen contents in magnet until 24 hours however the Dy-oxide content became 0.16- 0.17% in all scrap sizes after 48 hours.
- The 200-300 microns sized scrap had the least volume fraction of Dy2Fe17 about 1.54% at 6 hours and 1000- 2000 microns sized scrap had the highest volume frac-tion of Dy2Fe17 about 2.51% at 6 hours as shown in Fig. 6(b). Dy2Fe17 remained 1% more in 1000-2000 microns sized scrap which has the smallest volumetric surface for decomposition of Dy2Fe17. Dy2Fe17 is decomposed by Mg as reaction time increases. Remained Dy2Fe17 by size of pulverized scrap followed volumetric surface for decomposition until 24hours but Dy2Fe17 remained 0.12- 0.13% at 48 hours for all the scrap sizes.
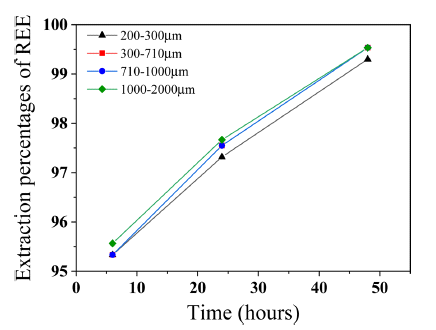
- Extraction percentages of REEs was calculated in Fig. 7 to confirm effect of decomposition and reduction behavior of Dy2Fe17 and Dy-oxide on Dy extraction. Extraction percentages of REEs from 200-300 microns sized scrap was 95.33% and from 1000-2000 microns sized scrap was 95.56% at 6 hours. Even if difference of extraction percentages between 200-300 microns and 1000-2000 microns sized scrap was only 0.23%, extraction percentages of Dy from 1000-2000 microns sized scrap was about 62% which was 2% higher extraction percentages of Dy than 200-300 microns sized scrap. Dy-oxide has 87.13 wt.% of Dy concentration and Dy2Fe17 has 25.5 wt.% of Dy concentration. Therefore, extraction percentages of REEs from 200-300 microns sized scrap which had the biggest Dy-oxide fraction was the least. Extraction rate of REEs from 200-300 microns sized scrap was the slowest and from 300-1000 microns sized scrap is the fastest as reaction time increases. Since Dy2Fe17 decomposition rate is faster than Dy-oxide reduction rate as shown in Fig. 6 hence extraction percentages of REEs from 300-2000 microns sized scrap was almost same at 48 hours about 99.53%. We can ascertain that the effect of Dy-oxide on Dy extraction is more compared to the Dy2Fe17. Therefore, size of pulverized scrap under 300 microns was not efficient for extraction on Dy due to highly remained Dy-oxide which prevents to extract Dy critically hence optimum size of pulverized scrap was over 300 microns to extract Dy. Finally, it is confirmed that smaller sized scrap has more phases including oxygen hence Dy-oxide is generated more and prevents Dy extraction.
3. Results and Discussion
Fig. 5
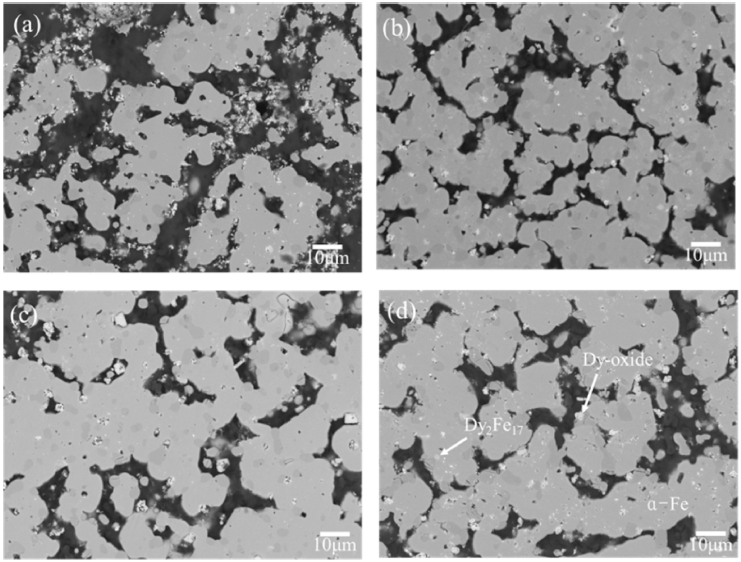
Microstructure of (a) 200-300 microns, (b) 300-710 microns, (c) 710-1000 microns, (d) 1000-2000 microns sized scrap after 48h LME process.

Fig. 6
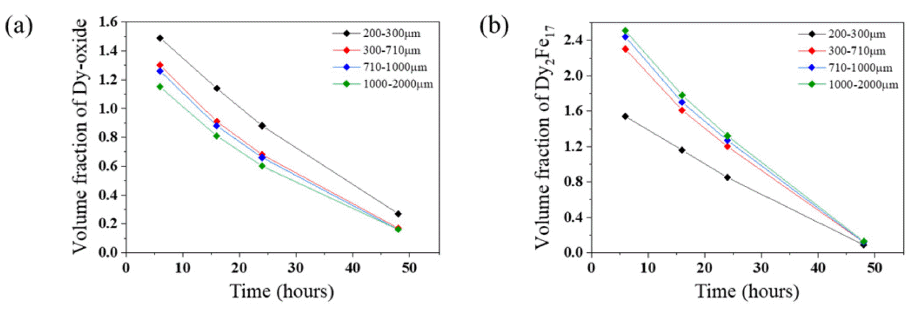
(a) Volume fraction of Dy-oxide and (b) Dy2Fe17 by size of pulverized scrap and reaction time.

- (Nd, Dy)-Fe-B magnet is easily oxidized during pulverization process due to high reactivity between REEs and oxygen. Oxygen contents was increasingly detected as the size of pulverized scrap decreases due to the increase in its volumetric surfaces. The 200-300 microns sized scrap had the highest oxygen contents about 2612 ppm and 1000-2000 microns sized scrap had the lowest oxygen contents about 1621 ppm. In the (Nd, Dy)-rich and (Nd, Dy)2Fe14B phases, phases including oxygen were appeared as (Nd, Dy)-oxide and (Nd, Dy)FeO3 phases from the results of microstructural and phase analysis. After LME process during 6 hours to 48 hours, Phases including oxygen disappeared except for the Dy-oxide which indicates the transformation of (Nd, Dy)-oxide and (Nd, Dy)FeO3 into Dy-oxide. Nd was not detected since extraction of Nd was completely proceeded. Dy was remained in the form of Dy2Fe17 and Dy-oxide. Volume fraction of Dy-oxide was the highest about 1.49% in the 200-300 microns sized scrap and the lowest about 1.15% at 1000-2000 microns sized scrap after 6 hours process. Since formation of phases including oxygen was more active with decreasing the size of pulverized scrap so the volume fraction of Dy-oxide was the highest in the smallest scrap size. Reduction of Dy-oxide contents by process time followed a trend of the oxygen contents in scrap until 24 hours however the Dy-oxide content in 300-2000 microns sized scrap reached at 0.16-0.17% at 48 hours. Volume fraction of Dy2Fe17 was the highest in 1000-2000 microns sized scrap due to its smaller surface for decomposition of Dy2Fe17. Decomposition of Dy2Fe17 by process time followed as trend similar to the volumetric surface until 24 hours however the Dy2Fe17 is remained about 0.12-0.13% after 48 hours. Dy-oxide has much Dy contents than Dy2Fe17 hence extraction percentages of REEs was the lowest from 200-300 microns sized scrap until 48 hours. In addition, decomposition rate of Dy2Fe17 is faster than reduction rate of Dy-oxide hence extraction percentages of REEs from 300-2000 microns sized scrap were same at the 48 hours about 99.53%. Therefore, size of pulverized scrap under 300 microns is inefficient for Dy extraction due to highly remained Dy-oxide hence optimum size of pulverized scrap has to be controlled over 300 microns for extracting Dy. Finally, it is confirmed that oxidation of magnet needs to be prevented in order to extract Dy since Dyoxide hinders Dy extraction more than the Dy2Fe17. Extraction percentages of rare earths using LME process in this study is 99% of extraction percentages of rare earths using hydro-metallurgical recycling [4-7]. However, any wastes are not generated with LME process compared to hydro-metallurgical recycling which uses chemicals for extract rare earths. Therefore, LME process is expected to be attractive alternatives of hydrometallurgical recycling with additional studies to optimize conditions for saving energy and improve extraction percentages of rare earths.
4. Conclusion
-
Acknowledgements
- This research was supported by a grant from the project “Development of environment friendly pyro-metallurgy process for high purity HREE and materialization” by the Korea Evaluation Institute of Industrial Technology (KEIT), Republic of Korea.
Acknowledgements
- 1. Y. S. Kim: M.S Thesis, A diffusion behavior of rare earth elements (Nd, Dy) from RE-Fe-B alloy by molten magnesium, University of Science and Technology, Daejeon (2017)..
- 2. Z. Samardžija, P. McGuiness, M. Soderžnik, S. Kobe and M. Sagawa: Mater. Charact., 67 (2012) 27..Article
- 3. T. Itakura, R. Sasai and H. Itoh: J. Alloys Compd., 408 (2006) 1382..Article
- 4. K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven,Y. Yang, A. Walton and M. Buchert: J. Clean Prod., 51 (2013) 1..Article
- 5. C. Tunsu, Y. Menard, D. Ø. Eriksen, C. Ekberg and M. Petranikova: J. Clean Prod., 218 (2019) 425..Article
- 6. A. C. Ni’am, Y. F. Wang, S. W. Chen, G. M. Chang and S. J. You: Chem. Eng. Process., 148 (2020) 107831..Article
- 7. N. Swain and S. Mishra: J. Clean Prod., 220 (2019) 884..Article
- 8. L. Omodara, S. Pitkäaho, E. M. Turpeinen, P. Saavalainen, K. Oravisjärvi and R. L. Keiski: J. Clean Prod., 236 (2019) 117573..Article
- 9. O. Diehl, M. Schönfeldt, E. Brouwer, A. Dirks, K. Rachut, J. Gassmann and O. Gutfleisch: J. Sustain. Met., 4 (2018) 163..Article
- 10. E. G. Polyakov and A. S. Sibilev: Metallurgist., 59 (2015) 368..Article
- 11. S. W. Nam, S. M. Park, D. H. Kim and T. S. Kim: Met. Mater. Int., (2020) 1..
- 12. S. M. Park, S. W. Nam, J. Y. Cho, S. H. Lee, S. K. Hyun and T. S. Kim: Arch. Metall. Mater., 65 (2020) 1281..
- 13. T. Akahori, Y. Miyamoto, T. Saeki, M. Okamoto and T. H. Okbae: J. Alloys Compd., 703 (2017) 337..Article
- 14. M. Firdaus, M. A. Rhamdhani, Y. Durandet, W. J. Rankin and K. McGregor: J. Sustain. Met., 2 (2016) 276..Article
- 15. H. J. Chae: ph. D. Dissertation, A study on the extraction of neodymium from Nd-Fe-B permanent magnets using liquid magnesium, Hanyang University, Seoul (2013)..
Figure & Data
References
Citations
Citations to this article as recorded by 

- Manipulation of reactivity based on metallic adsorption in magnesium alloy scraps for rare-earth recycling by liquid metal extraction
Sangmin Park, Yoonhyung Keum, Jaeyun Jeong, Seunghun Cha, Ju-Young Cho, Hyunchul Kim, Jiseong Lee, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Journal of Alloys and Compounds.2025; 1022: 178711. CrossRef - A Review of the Current Progress in High-Temperature Recycling Strategies for Recovery of Rare-Earth Elements from Magnet Waste
Ali Zakeri, Leili Tafaghodi
Journal of Sustainable Metallurgy.2025; 11(1): 88. CrossRef - Selective growth of Nb–Fe–B intermetallic compounds for the direct separation of rare earths based on manipulating liquation
Sangmin Park, Jaeyun Jeong, Seunghun Cha, Yoonhyung Keum, Ju-Young Cho, Hyungbeen Park, Taek-Soo Kim, Dae-Kyeom Kim, Myungsuk Song
Materials Today Sustainability.2024; 28: 101042. CrossRef - Separation and recovery Nd and Dy from Mg-REEs alloy by vacuum distillation
Sangmin Park, Dae-Kyeom Kim, Jaeyun Jeong, Jae Hong Shin, Yujin Kang, Rongyu Liu, Taek-Soo Kim, Myungsuk Song
Journal of Alloys and Compounds.2023; 967: 171775. CrossRef - The Supported Boro-Additive Effect for the Selective Recovery of Dy Elements from Rare-Earth-Elements-Based Magnets
Sangmin Park, Dae-Kyeom Kim, Javid Hussain, Myungsuk Song, Taek-Soo Kim
Materials.2022; 15(9): 3032. CrossRef - Influence of Dysprosium Compounds on the Extraction Behavior of Dy from Nd-Dy-Fe-B Magnet Using Liquid Magnesium
Sun-Woo Nam, Sang-Min Park, Mohammad Zarar Rasheed, Myung-Suk Song, Do-Hyang Kim, Taek-Soo Kim
Metals.2021; 11(9): 1345. CrossRef
Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process

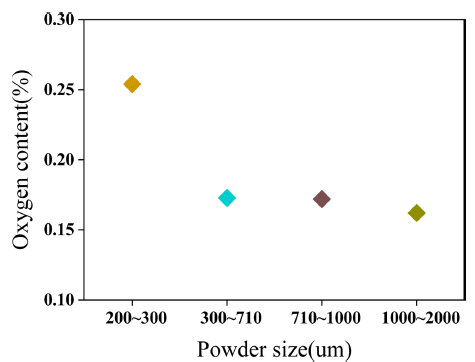

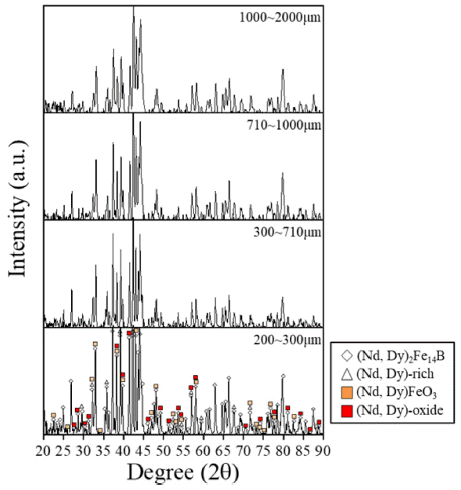


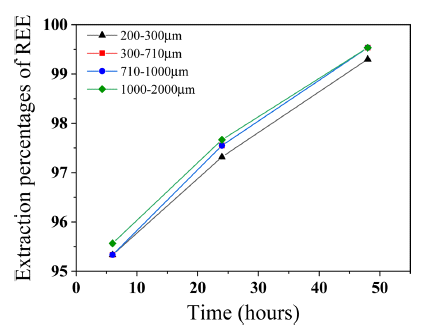
Fig. 1
Schematics of LME experiment.
Fig. 2
Oxygen contents by pulverized scrap.
Fig. 3
Microstructure of pulverized scrap and line scanning of oxygen.
Fig. 4
XRD analysis by size of pulverized scrap.
Fig. 5
Microstructure of (a) 200-300 microns, (b) 300-710 microns, (c) 710-1000 microns, (d) 1000-2000 microns sized scrap after 48h LME process.
Fig. 6
(a) Volume fraction of Dy-oxide and (b) Dy2Fe17 by size of pulverized scrap and reaction time.
Fig. 7
Extraction percentages by size of pulverized scrap and reaction time.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Effect of Oxidation Behavior of (Nd,Dy)-Fe-B Magnet on Heavy Rare Earth Extraction Process
Table 1
The composition of 39UH magnet
Table 1
TOP
 KPMI
KPMI


 ePub Link
ePub Link Cite this Article
Cite this Article







