Articles
- Page Path
- HOME > J Powder Mater > Volume 30(2); 2023 > Article
-
Article
- Study on Reaction Behavior of Mg-FeB Phase for Rare Earth Elements Recovery from End-of-life Magnet
- Sangmin Parka,b,†, Dae-Kyeom Kima,†, Rongyu Liua,c, Jaeyun Jeonga,d, Taek-Soo Kima,b, Myungsuk Songa,*
-
Journal of Korean Powder Metallurgy Institute 2023;30(2):101-106.
DOI: https://doi.org/10.4150/KPMI.2023.30.2.101
Published online: March 31, 2023
a Korea Institute for Rare Metals, Korea Institute of Industrial Technology, Incheon 21655, Republic of Korea
b Department of Industrial Materials and Smart Manufacturing Engineering, University of Science and Technology, Daejeon 34113, Republic of Korea
c College of Physics and Materials, Nanchang University, Nanchang 330031, China
d Department of Materials Science and Engineering, Inha University, Incheon 22212, Republic of Korea
- * Corresponding Author: Myungsuk Song, TEL: +32-226-1356, FAX: +32-226-1356, E-mail: mssong@kitech.re.kr
-
- S. Park·J. Jeong: 학생, D.-K. Kim: 연구원, R. Liu: 학생, T.-S. Kim: 수석연구원, M. Song: 선임연구원
†These authors contributed equally to this work
• Received: April 12, 2023 • Revised: April 21, 2023 • Accepted: April 23, 2023
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,209 Views
- 4 Download
Abstract
- Liquid metal extraction (LME), a pyrometallurgical recycling method, is popular owing to its negligible environmental impact. LME mainly targets rare-earth permanent magnets having several rare-earth elements. Mg is used as a solvent metal for LME because of its selective and eminent reactivity with rare-earth elements in magnets. Several studies concerning the formation of Dy-Fe intermetallic compounds and their effects on LME using Mg exist. However, methods for reducing these compounds are unavailable. Fe reacts more strongly with B than with Dy; B addition can be a reducing method for Dy-Fe intermetallic compounds owing to the formation of Fe2B, which takes Fe from Dy-Fe intermetallic compounds. The FeB alloy is an adequate additive for the decomposition of Fe2B. To accomplish the former process, Mg must convey B to a permanent magnet during the decomposition of the FeB alloy. Here, the effect of Mg on the transfer of B from FeB to permanent magnet is observed through microstructural and phase analyses. Through microstructural and phase analysis, it is confirmed that FeB is converted to Fe2B upon B transfer, owing to Mg. Finally, the transfer effect of Mg is confirmed, and the possibility of reducing Dy-Fe intermetallic compounds during LME is suggested.
- Liquid metal extraction (LME) process targeted to permanent magnet which has the highest contents of rare earths among their applications has been observed actively in transition to a greener economy for recovering rare earths [1-4]. Environmental damages on recovering rare earths don’t exist since LME process uses magnesium (Mg) as solvent metal without chemicals [5]. In fact, neodymium (Nd) which is mainly included in permanent magnet diffuses fast to Mg within 6 hours, hence eminent effect of LME process on recovering rare earths is proved [6-10]. However, low recovery efficiency of dysprosium (Dy) which mainly co-exists with Nd in permanent magnet is obviously low compared to hydro-metallurgical recycling process [11-13]. Researches on hindrance for recovering Dy from LME process using Mg were reported that debasement of recovery efficiency was caused by formation of Dy-Fe intermetallic compounds and Dy-oxide. In order to improve recovery efficiency of Dy, some researchers assessed thermodynamic calculation and controlled process parameters to reduce Dy-Fe intermetallic compounds and Dy-oxide [14-16]. It was confirmed that Dy-Fe intermetallic compounds were decomposed and Dy-oxide was reduced [14-16]. However, decomposition rate and reduction rate were still slow to improve recovery efficiency.
- In order to overcome the former problem, evaluation on input of additives for removing hindrance is needed to be considered. Boron (B) can be attractive additives for removing Dy-Fe intermetallic compounds, which are one of hindrance to recover Dy with reason as below. Previous researches on LME process were equally mentioned that B in permanent magnet during LME process was remained as Fe2B [14-16]. It means that B has the strongest affinity with Fe than any other elements in permanent magnet [17, 18]. Therefore, extraction of Dy to Mg can be effectively originated with B addition by forming Fe2B which can take Fe from Dy-Fe intermetallic compounds due to the strongest stability during LME process. However, B metals are not to be decomposed by Mg due to its strong affinity between B atoms and it is hard to fabricate. Therefore, it is needed to find other candidates which can be decomposed by Mg for supplying B to permanent magnet.
- In this study, we found that FeB alloy can be one of candidates since it can be decomposed by Mg through thermodynamic calculation and empirical experiment. This study shows effect of Mg on conveying B to permanent magnet through decomposition of FeB with microstructural aspect and phase analysis.
1. Introduction
- FeB alloy including B content of 16.2 wt.% was used as raw material as indicated composition in Table 1. The FeB alloy was pulverized with jaw crusher over 1000 microns and analysis was based on the partially reacted specimen to confirm decomposition of FeB after 24 hours. In order to confirm microstructures and phases, back scattered electrons mode (BSE) of scanning electron microscope (FE-SEM, Model JSM-100F) and X-ray diffraction (XRD, Model D/Max 2500PC) were observed. In order to simulate reaction behavior before experiment, ternary phase diagram at 900°C was calculated by Factsage 7.1 [19].
- After microstructural analysis of FeB alloy and thermodynamic calculations for simulating reaction behavior, reaction experiment between Mg and FeB was processed under optimized conditions for LME which were set as process temperature at 900°C and process time during 24 hours [14-16]. Input ratio between Mg and FeB alloy was set as 1:1 weight ratio which can accomplish phase decomposition from FeB to Fe2B based on thermodynamic calculations. Atmosphere of reaction experiment was under high purity Ar after vacuum about 10-3 torr for preventing oxidation of Mg. Reaction experiment was observed in a high frequency induction furnace (Model DTIH-0020VMF) which helps diffusion of B from FeB alloy to Mg. Schematics of reaction experiment is indicated in Fig. 1. In order to observe microstructure, BSE mode and energy dispersive X-ray analysis (EDS) of FESEM were used. In addition, bulk analysis mode of XRD with changing slit size was used for phase analysis to detect specific area after observing microstructure. Finally, effect of Mg on supplying B was obseved by analysis as mentioned above.
2. Experimental
- Thermodynamic calculation was assessed for simulating reaction behavior as shown in Fig. 2. As shown in Fig. 2, stability of Fe2B is the most stable among intermetallic compounds at 900°C by B addition. In addition, it is confirmed that MgB2 is more stable than FeB since MgB2 is formed after complete formation of Fe2B phases with enough B contents. Therefore, possibility of effect of Mg for conveying B from FeB was confirmed due to decomposition from FeB to Fe2B in reaction due to its higher stability under optimized LME conditions. Accordingly, input ratio between Mg and FeB could be set in zone of complete decomposition from FeB to Fe2B as indicated in Fig. 2 noticing with red circle. MgB2 can be applied as obstacle due to solidification for conveying B to magnet even if formation of MgB2 can be useful information for proving B diffusion from decomposition of FeB in this study. In fact, solidification of MgB2 is not expected to be appeared during LME process because B remains as liquid phase due to existence of Fe to form Fe2B as shown in Fig. 2. However, thermodynamic calculation is based on equilibrium status without considering process time hence reaction experiment was proceeded to get empirical data under optimized LME conditions.
- Prior to experiments, FeB alloy has to be confirmed whether it is homogeneous for applying reaction experiments to observe decomposition of FeB alloy by Mg. FESEM is applied for observing microstructural investigation as indicated in Fig. 3(a). In addition, phase analysis using XRD is modified with bulk mode to detect specific area noticing as red circle in cross section of raw material for confirming homogeneity as shown in Fig. 3(b). As a result of observing microstructure and phase of FeB alloy, any other phases excluding FeB were not observed.
- Reaction experiment at 900°C during 24 hours was proceeded to confirm phase decomposition. Microstructure was observed to confirm phase decomposition using FE-SEM as indicated in Fig. 4. Phase decomposition of FeB was not detected in normal FE-SEM as shown in Fig. 4(a) since difference of B contents between FeB and Fe2B is small. Therefore, BSE mode was used for distinguish each phase as shown in Fig. 4(b). There were 3 zones which colored with black, dark grey and light gray in reacted FeB alloy as shown in Fig. 4(b) and it means that FeB alloy reacted with Mg. Therefore, microstructural and phase analysis has to be observed in detail by each zone.
- In order to confirm microstructure of light grey and dark grey zone, EDS of FE-SEM was observed as shown in Fig. 5(a). Difference of composition in phases can be distinguished with Fe contents. It is confirmed that dark grey phase has 82.99 wt.% of Fe composition and light grey phase has 90.66 wt.% of Fe composition. Phase analysis by each zone was observed using XRD with bulk mode to detect specific region as shown in Fig. 5(b), (c). Zones were separated into dark grey phase dominated zone and light grey phase dominated zone. However, 1mm slit was used hence Mg matrix was also detected. FeB peaks mainly existed in dark grey phase dominated zone and Fe2B peaks mainly existed in light grey phase dominated zone. Through this result, it was found that light grey phase was Fe2B and dark grey phase was FeB. It means that B in FeB was diffused out and turned to be Fe2B. However, black phases in FeB alloy are not detected due to relatively less contents and the limit of slit size in bulk XRD. Therefore, microstructure of black phase was detected with EDS-mapping as indicated in Fig. 6. Black phase was composed with B and Mg as shown in Fig. 6(b). As shown in Fig. 6(b), location of Mg and B was highly similar with each other. In addition, Fe was included in alloy without black phases. As results of Fig. 5 and Fig. 6, we can confirm that B in FeB was diffused out to Mg with forming Mgboride and FeB phase was turned out Fe2B.
- Finally, it offers the clues that FeB can be decomposed to Fe2B with conveying B to Mg hence effect of Mg on supplying B was confirmed. In addition, FeB can be considered as possible additive for LME to reduce Dy-Fe intermetallic compounds due to conveying effect of Mg.
3. Results and Discussion
Fig. 2
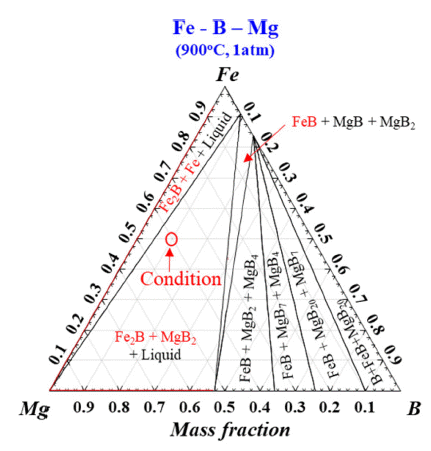
Mg-Fe-B ternary phase diagram at 900°C by Factsage noticing composition for experiments as red circle

Fig. 3
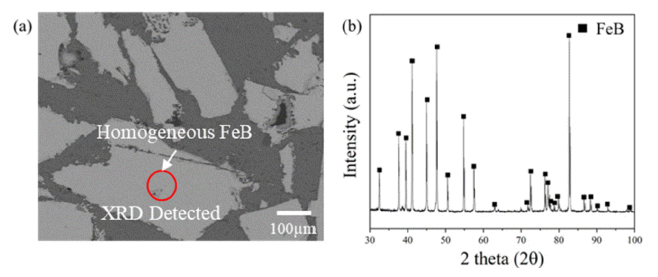
Observation on FeB raw materials with (a) Microstructure investigation using FE-SEM and (b) Phase analysis by XRD modified with bulk mode detecting specific area as indicated with red circle.

Fig. 4

Microstructure observation of FeB alloy by (a) normal SEM (b) BSE mode after reaction experiment at 900°C during 24 hours which condition is optimized for LME process.

Fig. 5
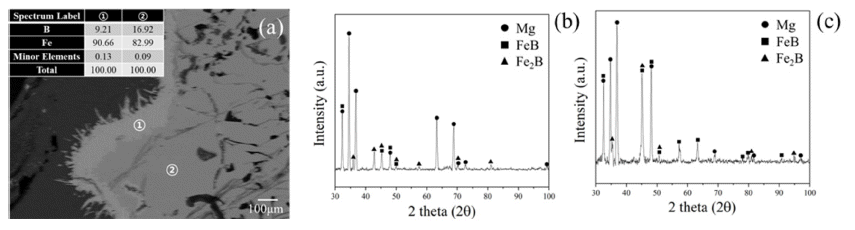
(a) Microstructure with SEM-EDS compositional analysis of light grey surface shown as ① and dark grey body of FeB indicated as ②, (b) phase analysis with XRD modified with bulk mode detecting dominated zone with ① and (c) dominated zone with ②.

- The thermodynamic calculations in this study indicate that FeB alloy can be used as an adequate additive to supply B using Mg. To confirm the decomposition of FeB, particles of over 1000 microns were selected, and the microstructure and phase analysis were carried out on the partially diffused specimen instead of a fully diffused one. The ternary phase diagram of Mg-Fe-B reveals that Fe2B and MgB2 are formed earlier than FeB as the B content increases, indicating that B in FeB can be diffused to Mg for conveyance to the magnet. MgB2 can be solidified with excess B in this study even though it is not formed in LME process due to its weaker stability than Fe2B. To confirm the empirical phase decomposition, a reaction experiment was conducted under optimized LME conditions in a high-frequency induction furnace. The FeB alloy was confirmed to be homogeneous by the BSE mode of FE-SEM and XRD analysis. After the reaction experiment, the microstructural and phase analysis were observed for confirming reaction behavior in ternary system. It was found that three zones where is composed with Fe2B, MgB2 and FeB were formed by the reaction. Accordingly, results explored that FeB can be decomposed to Fe2B with the diffusion of B to Mg. Finally, the conveying effect of Mg for B from FeB to the magnet was confirmed. Based on these clues, it is suggested that FeB alloy can be applied in the LME process as an additive. B is formed as Fe2B during the LME process due to its high affinity with Fe, which can react with Dy, making B a superior element to prevent the formation of Dy-Fe intermetallic compounds. FeB is evaluated as a suitable additive with a conveying effect of Mg from this research, instead of B metal, which cannot be easily decomposed by Mg. Therefore, FeB can be suggested as an additive for the LME process to improve the recovery efficiency of Dy and reduce Dy-Fe intermetallic compounds.
4. Conclusion
-
Acknowledgements
- This work was supported by Korea Institute of Industrial Technology as “International cooperation on the development of metallic compounds separation/recovery and byproducts materialization for total recycling during eco-friendly process” project (No. kitech JE-230013) and “Based on the utilization of waste resources, the highadded value and development of rare earth metal recovery technology through pyrometallurgical extraction for ecosystem conversion” project (No. kitech UR230006) and supported UST Young Scientist+ Research Program 2022 through the University of Science and Technology (No. 2022YS28).
Acknowledgement
- 1. K. Binnemans, P. McGuiness and P. T. Jones: Nat. Rev. Mater., 6 (2021) 459. .Article
- 2. K. Binnemans, P. T. Jones, T. Müller and L. Yurramendi: J. Sustain. Metall., 4 (2018) 126. .Article
- 3. H. Nakamura: Scr. Mater., 154 (2018) 273. .Article
- 4. B. Sprecher, Y. Xiao, A. Walton, J. Speight, R. Harris, R. Kleijn and G. J. Kramer: Environ. Sci. Technol., 48 (2014) 3951. .ArticlePubMed
- 5. K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y Yang, A. Walton and M. Buchert: J. Clean. Prod., 51 (2013) 1. .Article
- 6. Y. S. Kim, S. W. Nam, K. T. Park and T. S. Kim: Sci. Adv. Mater., 9 (2017) 2166. .Article
- 7. T. Akahori, Y. Miyamoto, T. Saeki, M. Okamoto and T. H. Okabe: J. Alloys. Compd., 703 (2017) 337. .Article
- 8. H. W. Na, Y. H. Kim, H. T. Son, I. H. Jung, H. S. Choi and T. B. Kim: Curr. Nanosci., 10 (2014) 128. .Article
- 9. T. H. Okabe, O. Takeda, K. Fukuda and Y. Umetsu: Mater. Trans., 44 (2003) 798. .Article
- 10. Y. Xu, L. S. Chumbley and F. C. Laabs: J. Mater. Res., 15 (2000) 2296. .Article
- 11. Y. Zhang, F. Gu, Z. Su, S. Liu, C. Anderson and T. Jiang: Metals., 10 (2020) 841. .Article
- 12. M. Gergoric, A. Barrier and T. Retegan: J. Sustain. Metall., 5 (2019) 85. .Article
- 13. D. Kim, L. E. Powell, L. H. Delmau, E. S. Peterson, J. Herchenroeder and R. R Bhave: Environ. Sci. Technol., 49 (2015) 9452. .ArticlePubMed
- 14. S. W. Nam, S. Park, M. Z. Rasheed, M. S. Song, D. H. Kim and T. S. Kim: Metals., 11 (2021) 1345. .Article
- 15. S. Park, S. W. Nam, J. Y. Cho, S. H. Lee, S. K. Hyun and T. S. Kim: Arch. Metall. Mater., 65 (2020) 1281. .
- 16. S. Park, S. W. Nam, S. H. Lee, M. S Song and T. S. Kim: J. Korean Inst. Met. Mater., 28 (2021) 91. .Article
- 17. V. Koç: Mater. Res. Express., 6 (2020) 1265c3. .Article
- 18. L. G. Yu, X. J. Chen, K. A. Khor and G. Sundararajan: Acta Mater., 53 (2005) 2361. .Article
- 19. G. Profeta, A. Continenza, F. Bernardini and S. Massidda: Phys. Rev. B., 66 (2002) 184517..Article
Figure & Data
References
Citations
Citations to this article as recorded by 

Study on Reaction Behavior of Mg-FeB Phase for Rare Earth Elements Recovery from End-of-life Magnet
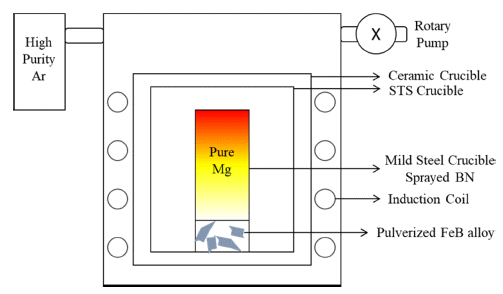


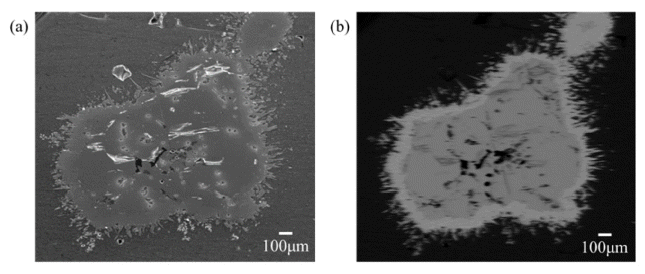
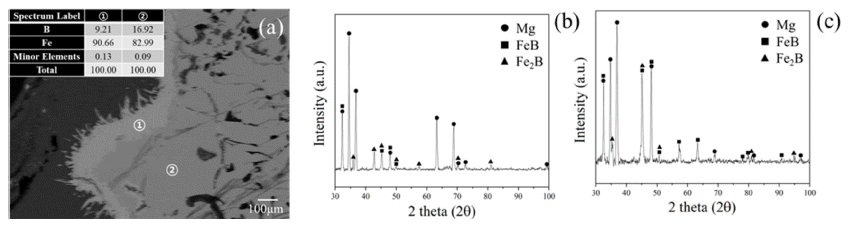
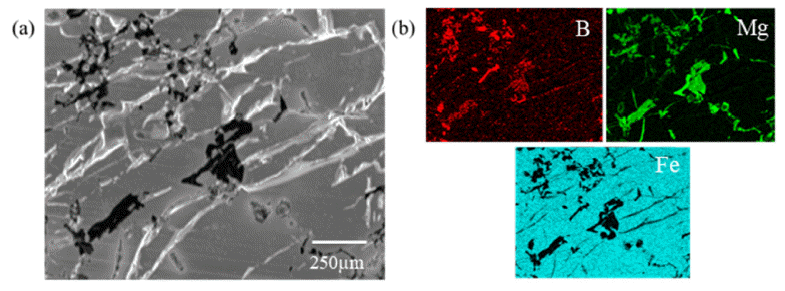
Fig. 1
Schematics of Reaction Experiment.
Fig. 2
Mg-Fe-B ternary phase diagram at 900°C by Factsage noticing composition for experiments as red circle
Fig. 3
Observation on FeB raw materials with (a) Microstructure investigation using FE-SEM and (b) Phase analysis by XRD modified with bulk mode detecting specific area as indicated with red circle.
Fig. 4
Microstructure observation of FeB alloy by (a) normal SEM (b) BSE mode after reaction experiment at 900°C during 24 hours which condition is optimized for LME process.
Fig. 5
(a) Microstructure with SEM-EDS compositional analysis of light grey surface shown as ① and dark grey body of FeB indicated as ②, (b) phase analysis with XRD modified with bulk mode detecting dominated zone with ① and (c) dominated zone with ②.
Fig. 6
(a) Microstructure and (b) EDS-mapping result of black phase in FeB alloy.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Study on Reaction Behavior of Mg-FeB Phase for Rare Earth Elements Recovery from End-of-life Magnet
Table 1
The composition of FeB alloy
Table 1
TOP
 KPMI
KPMI



 Cite this Article
Cite this Article






