Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 23(6); 2016 > Article
-
ARTICLE
- Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
- A-Young Lee, Gwang-Yeob Leea, Hye-Ryeong Oh, Hyeon-Ah Kim, Song-Yi Kim, Min-Ha Lee*
-
Journal of Korean Powder Metallurgy Institute 2016;23(6):409-413.
DOI: https://doi.org/10.4150/KPMI.2016.23.6.409
Published online: November 30, 2016
Advanced Functional Materials R&D Group, Korea Institute of Industrial Technology, Incheon 21999, Korea
a Advanced Analysis Center, Korea Institute of Science and Technology, Seoul 02792, Korea
- *Corresponding author : Min-Ha Lee, +82-32-850-0424, +82-32-850-0304, mhlee1@kitech.re.kr
• Received: November 7, 2016 • Revised: November 20, 2016 • Accepted: November 23, 2016
© The Korean Powder Metallurgy Institute. All rights reserved.
- 928 Views
- 1 Download
Abstract
- Electrical wire explosion in liquid media is a promising method for producing metallic nanopowders. It is possible to obtain high-purity metallic nanoparticles and uniform-sized nanopowder with excellent dispersion stability using this electrical wire explosion method. In this study, Ni-Fe alloy nanopowders with core-shell structures are fabricated via the electrical explosion of Ni-Fe alloy wires 0.1 mm in diameter and 20 mm in length in de-ionized water. The size and shape of the powders are investigated by field-emission scanning electron microscopy, transmission electron microscopy, and laser particle size analysis. Phase analysis and grain size determination are conducted by X-ray diffraction. The result indicate that a core-shell structured Ni-Fe nanopowder is synthesized with an average particle size of approximately 28 nm, and nanosized Ni core particles are encapsulated by an Fe nanolayer.
- The electrical wire explosion (EWE) method is a promising method for producing metallic nanoparticles. Highpurity metallic nanoparticles with a uniform size distribution can be produced by EWE, where the metal or alloy wire is exploded using high pulsed current and voltage during the EWE process [1, 2]. It has been established that the average particle size of nanosized powders synthesized in this manner increases with increasing atmospheric gas pressure during synthesis, as the increased atmospheric pressure suppresses the expansion of the metallic vapor present during synthesis [3-5].
- To produce smaller nanoparticles or those with more narrow size distributions using EWE, the medium around the wire should have high-density inert properties to suppress the formation of plasma, be able to expand to support the vapor volume present, and have a large heat capacity for rapid cooling of the vapor to prevent particles from being grown through collisions. These conditions are critical parameters of the EWE process and could be satisfied by using a liquid medium [6]. Liquid media have high density around the wire, adequate space is created in the liquid during the explosion by shock pressure, and the vapor and particles can be quickly cooled due to the large heat capacity of the medium [1].
- There are several important advantages of liquid EWE processes compared to conventional wire explosions in air. A non-oxide metal powder can be produced without vacuum processing due to the shielding effect of the medium, and the non-oxide phase can be safely kept in the final stage for eventual applied use. Another important advantage is the homogeneous distribution of the nanoparticles in dispersive liquids such as binder material for the fabrication of laminate electrodes [5]. Several previous reports have detailed the synthesis of alloy nanoparticles by EWE processing, such Cu-Ni, Cu-Zn and Al-Cu systems [7-9]. However, the detailed formation mechanism of the alloyed nanoparticles, and control over the composition and characteristics of the resulting nanoparticles, are still not well understood due to the complexity of the EWE process [7].
- Ni-Fe bimetallic powders are widely used for various applications such as in catalysts and soft magnetic materials [10, 11]. In addition to the synthesis of nearly spherical FeNi3 intermetallic compound particles by EWE, Ni-Fe bimetallic alloy nanoparticles have been produced from Ni-plated Fe wires by a pulsed wire discharging method [12, 13]. In this study, we investigate the formation of Ni- Fe bimetallic nanoparticles with core-shell structures created by EWE of Ni-Fe alloy wires in de-ionized water. The effects of processing parameters on the particle size, dispersibility and structure are also presented.
Introduction
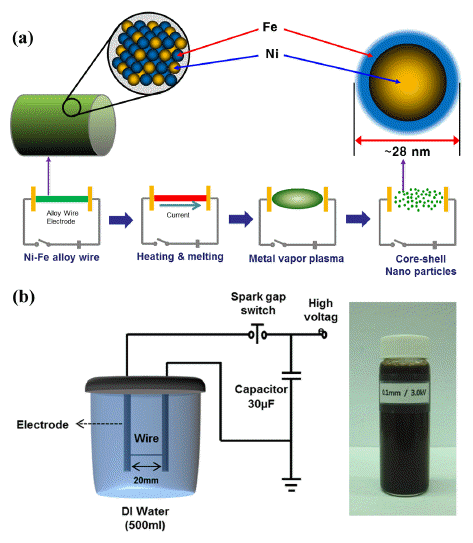
- The nickel-iron alloyed nanopowders were fabricated by EWE in liquid media using Ni-42 wt% Fe alloy wires 0.1 mm in diameter. A solid Ni-Fe alloy wire was placed between EWE electrodes with 20 mm interval lengths and a high current density was passed through (~1010 A/ m2) in a very short time period (~106 s). Schematic diagrams of the overall experimental process and the equipment setup for EWE are shown in Fig. 1. The Ni-Fe alloy wire, as the electrode material, was placed in a cylinder filled with 500 mL de-ionized water. Typical experimental parameters are summarized in Table 1. The size and morphology of the resulting powders were characterized by field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM) and laser particle size analysis (LPSA). Phase analysis was conducted by X-ray diffraction (XRD) with Co-Kα radiation. The elemental concentration was determined by energy dispersive spectroscopy (EDS).
Experimental
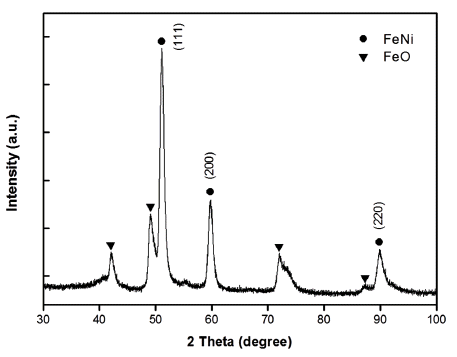
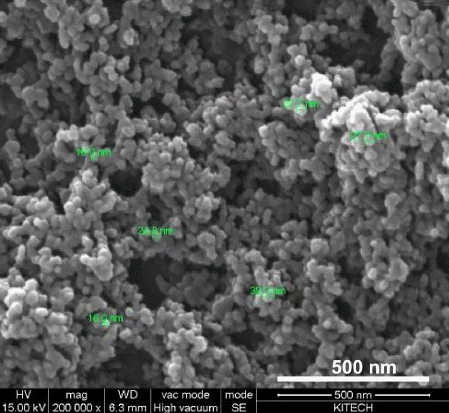
- XRD analysis of the dried Ni-Fe powders was performed in order to investigate the major phase of the synthesized nanopowders. XRD patterns obtained from the synthesized Ni-Fe powders are shown in Fig. 2. The sharp peaks diffracted from the crystalline phases of powders correspond to the diffraction data in the JCPDS cards of Fe-Ni (JCPDS 47-1405) and FeO (JCPDS 01- 1223). The extra peak observed in Fig. 2 originates from the oxide layer (FeO) on the particle surface, which forms during drying. The Ni-Fe nanopowder morphology observed by FE-SEM (Fig. 3) is a typical homogeneous distribution of spherical small particles. Moreover, a majority of the particles strongly agglomerated during drying.
- TEM investigations were performed to evaluate the detailed microstructures of the nanopowder, as shown in Fig. 4. Bright field TEM images shown in Fig. 4(a) indicate that the particles are nearly spherical in shape, and most particles are less than 30 nm in size. These particles also have a size range over 20 nm and have clear core-shell structures, as shown in Fig. 4(b).
- The elemental concentration of the core-shell structured nanopowder was determined by EDS in conjunction with TEM, with the results summarized in Table 2. The EDS results show that the powder consists of a Nirich Fe solid solution core (region A) and a FerichNi solid solution shell (region B). However, the stoichiometry of Ni and Fe in those solid solutions does not match with the stable phases in binary Ni-Fe systems or the initial Ni-Fe alloy wire due to the extreme conditions during EWE processing. For this reason, the XRD diffraction pattern in Fig. 2 only indicates that the powders consist of Ni50Fe50 solid solution (single-phase) due to the resolution limits of conventional laboratory X-rays.
- Based on the EDS analysis (Table 2), the concentration of Ni in the powder clearly differs between the core and shell regions. The majority of the Ni is concentrated at the core (72.13 wt%; region A) rather than in the shell (5.76 wt%; region B). However, the concentration deviation of Ni is not significant in the smallest particles (39.97 wt% in region C), which arise due to the immature development of core-shell structures by rapid solidification during EWE. Small amounts of oxygen are also present in the shell region. The chemical composition of the particle is different from the initial Ni-Fe alloy wire due to the different vaporization enthalpy values of Ni and Fe, as shown in Table 3, as well as the encapsulation of Ni particle by the Fe layer.
- The crystalline particle size can be calculated by using Scherrer’s formula [14],
- where the wavelength of X-rays for Co-Kα radiation is λ = 1.7902 Å, and B is the full-width-half-maximum at the peak for 2θB. The mean crystalline particle size from the XRD diffraction data was estimated to be approximately 20 nm based on equation (1). This estimated value is wellmatched with the TEM results shown in Fig. 4.
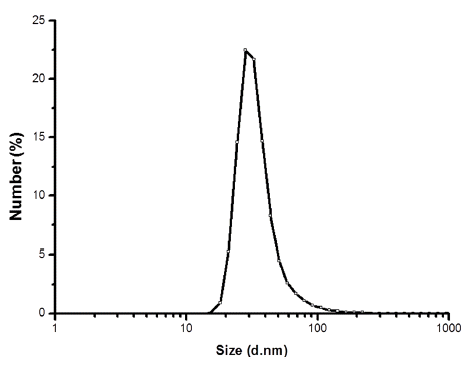
- Fig. 5 shows the particle size distribution of powders obtained from LPSA. The average particle size is approximately 28 nm, which is similar to the results obtained by TEM and calculated from Scherrer’s formula. There are no secondary peaks arising from micrometer-sized liquid droplets originated from incomplete vaporization or agglomeration of particles [15], which indicates that the discharge energy of the EWE process is enough to vaporize the entire Ni-Fe wire.
- Based on the thermodynamic data of Ni and Fe, the formation of these alloy nanoparticles can be considered to involve three stages: over-heating of the wire, the explosion process, and condensation [7]. The wire is rapidly heated and melts due to a pulsed high-intensity current. The melted metal is further heated by the ever increasing current density due to increasing resistance, which leads to evaporation of the material and subsequent plasma formation. The explosion occurs and nanosized powders are dispersed in the medium at high speeds. The velocity of powder expansion and the system temperature may exceed 1,000 m/s and 10,000 K, respectively [12].
Results and Discussion
Fig. 4
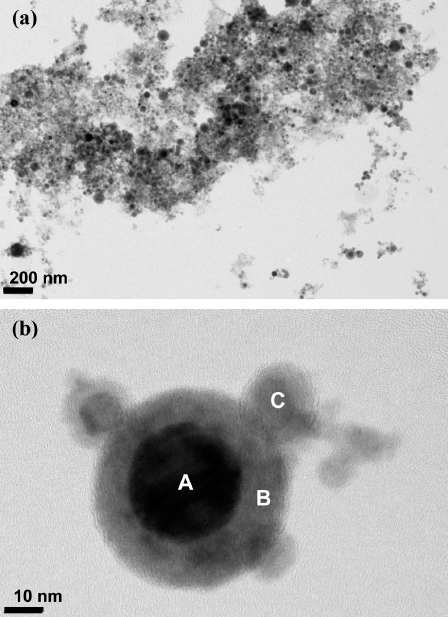
TEM images showing (a) the overall morphology with detail microstructures of Ni-Fe nanopowder, and (b) core-shell nanopowder structures from EDS analysis.

Table 2
Chemical composition of the core-shell structured nanopowder synthesized by EWE.
| Region | Ni (wt.%) | Fe (wt.%) |
|---|---|---|
|
|
||
| A | 72.13 | 27.87 |
| B | 5.76 | 94.24 |
| C | 39.97 | 60.03 |
Table 3
Physical properties of elemental starting materials.
| Material | Vaporization Energy (Ws, J/mm3) | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|
|
|
|||
| Ni | 53.9 | 1455 | 2913 |
| Fe | 55.4 | 1538 | 2862 |
| Ni-42Wt%Fe | - | 1407 | - |
- NiFe nanopowder was successfully synthesized by the electrical wire explosion of NiFe alloy wire in de-ionized water. The alloyed nanoparticles were nearly spherical in shape with a mean particle size of approximately 28 nm and a narrow size distribution range. TEM results also indicated that the particles have core-shell structures with partitioning between the constituent Ni and Fe elements. The particle core consisted of a Ni-rich phase while the shell featured an Fe-rich phase based on TEM and EDS result. This study shows that core-shell structured Ni-Fe bimetallic nanopowders can be synthesized from alloy wires via a simple and effective EWE method.
Conclusions
-
Acknowledgements
- This work was supported by the Industrial Strategic Technology Innovation Program (Project No. 20142020103910) funded from the Ministry of Trade, Industry and Energy (MOTIE) through the Korea Institute of Technology Evaluation and Planning (KETEP). Additional support was provided by the Civil-Military Technology Cooperation Program under Contract No. 14-BR-MA-05.
Acknowledgements
- 1. C Cho, Y W Choi, C Kang and G W Lee: Appl. Phys. Lett., (2007) 91 141501.
- 2. W B Kim, J S Park, C Y Suh, J C Lee, J H Kim and Y J Oh: J. Korean Powder Metall. Inst., (2007) 14 108.Article
- 3. Yatsui K Jiang W.: IEEE Trans. Plasma Sci., (1998) 26 1498.Article
- 4. C Cho, Y W Choi and W Jiang: J. Korean Phys. Soc., (2005) 47 987.
- 5. C Cho, K Murai, T Suzuki, H Suematsu, W Jiang and K Yatsui: IEEE Trans. Plasma Sci., (2004) 32 2062.Article
- 6. C H Cho, S H Park, Y W Choi and B G Kim: Surf. Coat. Technol., (2007) 201 4847.Article
- 7. Q Wang, H Yang, J Shi and G Zou: Mater. Sci. Eng. A Struct. Mater., (2001) 307 190.
- 8. W Kim, J S Park, C Y Suh, H Chang and J C Lee: Mater. Lett., (2007) 61 4259.Article
- 9. Y S Kwon, V V An, A P Ilyinb and D V Tikhonov: Mater. Lett., (2007) 61 3247.Article
- 10. A L Kustov, A M Frey, K E Larsen, T Johannessen, J K Nørskov and C H Christensen: Appl. Catal. A Gen., (2007) 320 98.Article
- 11. X Y Qin, J S Lee, J G Nam and B S Kim: Nanostruct. Mater., (1999) 11 383.Article
- 12. L H Bac, Y S Kwon, J S Kim, Y I Lee, D W Lee and J C Kim: Mater. Res. Bull., (2010) 45 352.Article
- 13. G H Park, G Y Lee, H A Kim, A Y Lee, H R Oh, S Y Kim, D H Kim and M H Lee: Mater. Sci. Eng. B Solid State Mater. Adv. Technol., (2016) 212 24.
- 14. Y R Uhm, W W Kim, S J Kim, C S Kim and C K Rhee: J. Appl. Phys., (2003) 93 7196.ArticlePDF
- 15. C Cho, Y Kinemuchi, H Suematsu, W Jiang and K Yatsui: Jpn. J. Appl. Phys., (2003) 42 1763.Article
References
Figure & Data
References
Citations
Citations to this article as recorded by 

Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
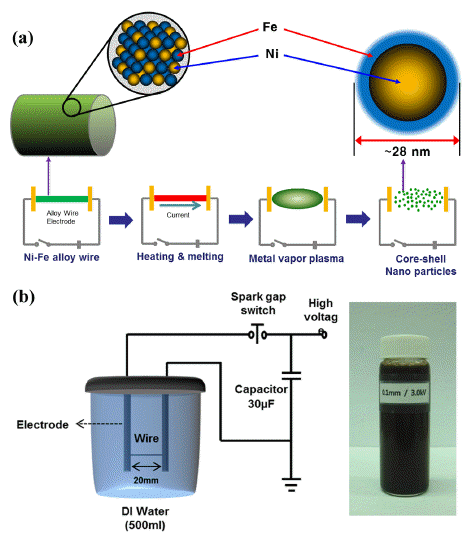

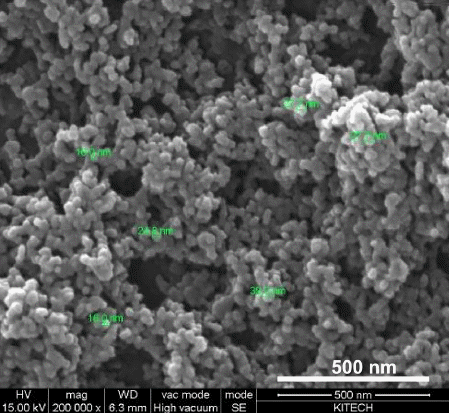

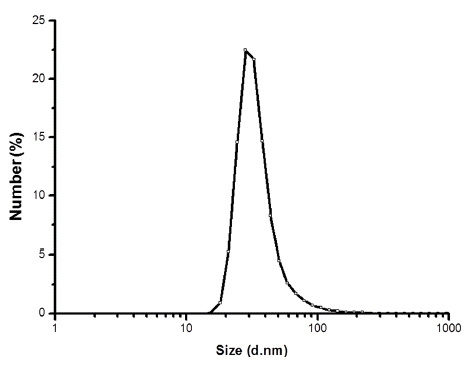
Fig. 1
Schematic diagram of (a) the experimental procedure and (b) EWE equipment, and synthesized Ni-Fe nanopowder dispersed in solution.
Fig. 2
XRD pattern obtained from Ni-Fe nanopowder.
Fig. 3
FE-SEM image showing the surface morphology of the Ni-Fe nanopowder.
Fig. 4
TEM images showing (a) the overall morphology with detail microstructures of Ni-Fe nanopowder, and (b) core-shell nanopowder structures from EDS analysis.
Fig. 5
Particle size distribution of the Ni-Fe nanopowder synthesized by EWE.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fabrication of Core-Shell Structured Ni-Based Alloy Nanopowder by Electrical Wire Explosion Method
| Wire diameter | 0.1 mm |
| Length of exploded wire | 20 mm |
| Applied voltage | 3.0 kV |
| Capacitance | 30 μF |
| Solution | De-ionized water |
| Region | Ni (wt.%) | Fe (wt.%) |
|---|---|---|
|
|
||
| A | 72.13 | 27.87 |
| B | 5.76 | 94.24 |
| C | 39.97 | 60.03 |
| Material | Vaporization Energy (Ws, J/mm3) | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|
|
|
|||
| Ni | 53.9 | 1455 | 2913 |
| Fe | 55.4 | 1538 | 2862 |
| Ni-42Wt%Fe | - | 1407 | - |
Table 1
Experimental parameters of the EWE process.
Table 2
Chemical composition of the core-shell structured nanopowder synthesized by EWE.
Table 3
Physical properties of elemental starting materials.
Table 1
Table 2
Table 3
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article





