Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 27(6); 2020 > Article
-
ARTICLE
- Interaction of Detonation Nanodiamonds with Hispidin
- Changkyu Rheea,*, Whungwhoe Kima, Andrey E. Burovb,c, Alexey P. Puzyrb, Vladimir S. Bondarb
-
Journal of Korean Powder Metallurgy Institute 2020;27(6):458-463.
DOI: https://doi.org/10.4150/KPMI.2020.27.6.458
Published online: November 30, 2020
a Nuclear Materials Research Division, Korea Atomic Energy Research Institute (KAERI), Daejeon 305-353, Republic of Korea
b Institute of Biophysics SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”, 660036 Krasnoyarsk, Russia
c Federal Research Center for Information and Computational Technologies, 660049 Krasnoyarsk, Russia
- *Corresponding Author: Changkyu Rhee, TEL: +82-42-868-8551, FAX: +82-42-868-8549, E-mail: ckrhee@kaeri.re.kr
- - C. Rhee, W. Kim, A. E. Burov, A. P. Puzyr and V. S. Bondar: 박사
• Received: November 19, 2020 • Revised: December 7, 2020 • Accepted: December 7, 2020
© The Korean Powder Metallurgy Institute. All rights reserved.
- 718 Views
- 1 Download
Abstract
- Hispidin is a secondary metabolite found in numerous medicinal mushrooms that has attracted significant attention, owing to its distinct biological effects, including antioxidant, anti-inflammatory, antitumor, and cytoprotective properties. Experiments are being carried out to study the interaction of detonation nanodiamonds (DNDs) with synthetic and natural hispidin sourced from extracts of Pholiota sp. fungus. The bioluminescence method is used to determine the adsorption/ desorption properties of DNDs toward hispidin. It is found that hispidin forms strong conjugates with DNDs, and the use of various eluents does not result in a significant release of the adsorbed hispidin molecules. DND-bovine serum albumin (BSA) complex, where DNDs serve as a carrier for the protein and the latter acts as a hispidin sorbent, has been developed and applied in hispidin adsorption/desorption tests. The results support the use of the DNDs as a carrier for hispidin in medical applications. They also advocate the application of the DND-BSA complex for isolating the substance from fungal extracts.
- Physicochemical properties of nanodiamonds produced by explosive synthesis define their wide applicability for biotechnological and biomedical purposes [1-3]. First of all, they include polymorphic and easily tailored surface, low toxicity and high biocompatibility. The results of various studies enabled to formulate several directions within which the application of detonation nanodiamonds for biological and medical purposes is currently under way. The main attention is focused on the use of DNDs in separation and purification of proteins, reusable indication and biochemical diagnostics systems, targeted delivery of biologically active substances, detoxification sorbents and development of therapeutics of combined and prolonged action [2-7].
- One of the topical areas of modern biotechnology is the discovery of new sources of natural raw materials for obtaining target products demanded in medicine, biology and pharmaceutical industries. In particular, in many countries of the Asia-Pacific region a vast research is undergoing to study the pharmacological activity and the practical use of compounds synthesized by basidiomycetes [8-10]. Among such compounds, hispidin has attracted a great deal of attention. Hispidin, a secondary metabolite presented in a number of edible and medicinal mushrooms, has been found to possess distinct biological effects, including antioxidant, anti-inflammatory, antitumor and cytoprotective properties. Hispidin (C13H10O5) fails under the class of phenolic compounds, its molecular weight is 246.21 g/mol. Studies of the properties of hispidin are carried out using the synthesized substance or that isolated from fungal extracts [11, 12].
- In this study the interaction of DNDs with synthetic and natural hispidin was investigated. Two goals were pursued: to check the applicability of DNDs as a sorbent for separation of hispidin from fungi and as a potential carrier in delivery system of this substance. It is known that hispidin interacts with a number of proteins [11, 12]. This fact motivated us to develop DND-BSA complex, where DND was a carrier for the protein while the latter acted as a sorbent for the hispidin molecules. Bovine serum albumin, a serum albumin protein derived from cows, has been widely used as a template to synthesize nanostructures [13]. To our knowledge, the result of this work is the first illustration of the use of DND for separation of hispidin from fungal extracts.
- A Pholiota species, which is widely distributed in temperate regions, was chosen as a natural source of hispidin. Majority of Pholiota sp. are edible and medicinal mushrooms, they are wood parasites. Compounds extracted from the fruiting bodies of Pholiota sp. display a variety of important biological activities, such as antitumor [14], anti-HIV-1and antioxidant activities [15, 16]. It was revealed that the fruiting bodies of Pholiota sp. contained hispidin [17].
- Determination of the hispidin content in fungal extracts is a complex and multi-stage procedure. The most commonly used method, which is based on recording the retention time on the columns in high-performance liquid chromatography, requires specialized equipment and expertise, as well as it is a time consuming process [18]. Recently [19], for a quantitative evaluation of hispidin in solutions we proposed the luminescent analysis method which is based on the fact that hispidin is involved in fungal bioluminescence as a precursor of luciferin [20]. The bioluminescence of fungi is found to be a two-stage process. First, hispidin is reduced by an NAD(P)Hdependent enzyme to a luciferin. In the second stage, the luciferin is oxidized by molecular oxygen under luciferase catalysis to emit visible light [20].
- Stability of the luminescent system isolated from luminous fungus Armillaria borealis and high sensitivity of the bioluminescent reaction allows carrying out multiple analyte detection with a limit of 1.3∙10-11 grams. A linear dependence of the luminescent response on hispidin in the concentration range of 5.4∙10-5 –1.4∙10-2 μM was shown [19]. Using the bioluminescence method allows determining the concentration of hispidin in solutions and analyzing the adsorption/desorption properties of DNDs towards the substance.
Introduction
- Nanodiamonds
- Detonation nanodiamonds (0-150 grade powder) acquired from Real-Dzerzhinsk LLC (Russia) was used in this study as the starting material. In order to remove surface impurities and improve colloidal stability, the starting DNDs were treated using a patented modification procedure [21]. A suspension was prepared by adding deionized water (Milli-Q system, Millipore U.S.A.) to the modified DND powders at concentration of 3 wt%. Fractionation was performed using a centrifuge 5415R (Eppendorf, Germany) to obtain two stock samples. The sample named “S” (small) was a supernatant collected after centrifugation of the DND suspension at 16000 g for 30 min at 10°C. The “L” (large) sample was the residue remained. The obtained samples were dried, weighed and stored at room temperature before use. Unimodal particle size distributions in water were determined with the dynamic light scattering technique using a Zetasizer Nano ZS (Malvern Instruments Ltd., UK). The data were averaged over 6 measurements, 30 runs each performed on 0.1 wt.% DND suspensions at 25°C. The measured average particle size of S fraction was 48.3 ± 4.2 nm, while L showed to be 342.7 ± 13.3 nm.
- In vitro luminescence system
- Finely crushed mycelium of Armillaria borealis (IBSO 2328 from CCIBSO 836 collection, IBP SB RAS, Russia) was used as the inoculum. Mycelium pellets were cultivated in 300-ml flasks containing 100 ml of nutrient medium (potato extract - 200 g/L, dextrose - 20 g/L) for about 20 days at 24°C under continuous agitation using a Max Q 4000 incubating shaker (Thermo Scientific, U.S.). Then mycelium was washed with water, scissored into pieces, placed into a cooled 0.1 M phosphate buffer solution (pH 7.0) containing 1% BSA (Serva, Germany) and homogenized ultrasonically (ultrasonic device Volna UZTA 0.63/22–0, Russia) in ice bath. The sonication was performed three times, 10 s each with 1 min interval. The homogenate was centrifuged at 40000 g for 30 min at 4°C (Avanti® J–E centrifuge, Beckman–Coulter, U.S.). The supernatant (cold extract) containing components of the luminescence system was placed into MCT-150-C microtubes (Axygen Scientific, Inc., U.S.) and frozen at - 80°C in a Sanyo Biomedical Freezer, model MDF – U333 (SANYO Electric Co., Ltd., Japan).
- Hispidin sources
- Synthetic hispidin acquired from Sigma-Aldrich, U.S. and hot extracts from Pholiota sp. were used as the hispidin sources. The hot extracts from Pholiota sp. were prepared as follows. Freshly harvested fruiting bodies were rinsed in distilled water to remove impurities, crushed in a mortar with pestle and transferred into a heat resistant glass. Deionized water was added to the biomass (1:4, water: biomass, v/v), then the sample was placed into the microwave oven (MW 712BR, Samsung, Malaysia) and heated until boiled. The sample was cooled on ice and centrifuged at 20000 g for 30 min at 4°C. The supernatant was filtrated through a 10 kDa membrane, freeze dried and stored at a temperature of -20°C. Just before use, the freezing was thawed and diluted 50 times with DI water.
- Luminescence measurement
- An aliquot of the cold extract (50 μl) in MCT-200-C microtubes was placed in a Glomax® 20/20 luminometer (Promega Bio Systems Sunnyvale, Inc., U.S.) and 2.5 μl 10 mM NADPH (Serva, Germany) was added to trigger the luminescent reaction. After the luminescence reached the constant level, 2.5–5 μl of the testing probe was injected and luminescence was monitored in realtime. Light emission was expressed as relative light units (RLU).
- DND-BSA complex
- Before the immobilization of BSA, the surface of DNDs was chemically activated with p-benzoquinone to provide sites for covalent bounding of the protein. The S fraction was used in these experiments. A phosphate buffer (20 mM, pH 8.0) and 100 mg of hydroquinone dissolved in 1 ml of aqueous ethanol (20%) were consequently added with stirring to an aliquot (5 ml) of DND suspension and the mixture was incubated for 2 h at room temperature under stirring. To remove unreacted components, DNDs were collected by centrifugation at 16000 g for 10 min at 10°C and the sediment was washed with DI water followed by several cycles of washing with 1 M NaCl, centrifugation and rinsing with water.
- BSA protein dissolved in 50 mM Na-bicarbonate buffer (рН 8.0) was mixed with the activated DND suspension (the protein to DNDs ratio - 1:2 (w/w)). The mixture was kept overnight at 4oС under continual stirring. DNDs with the immobilized protein were collected by centrifugation at 16000 g for 10 min at 10oС. The precipitate was repeatedly washed with NaCl solution (0.25M) to remove unbound proteins. The amount of protein in the supernatants was determined from the peak absorbance at 280 nm using the UV-1800 spectrophotometer. The measured adsorption capacity of DNDs towards BSA calculated as difference between the protein concentration in the initial BSA solution and supernatant was show to be 0.25 mg of protein per 1 mg of DNDs.
- Adsorption of hispidin on DNDs and DND-BSA complex
- To obtain DND-hispidin conjugates, 200 or 400 μl of 3 wt% DND suspension was added to 1 ml of aqueous solutions of synthetic hispidin (0.33 μM) into Eppendorf vials. The vials were shaken at ambient temperature for 10 min to ensure equilibrium adsorption. To precipitate DND particles, the mixture was centrifuged at 16000 g for 10 min at 10oС. The residue was washed by centrifugation with DI water in order to remove unbound hispidin molecules. The hispidin solution with no DNDs was used as the control sample.
- The DND-BSA complex suspension (about 0.5 mg of BSA immobilized on 2 mg of DNDs) or DND suspension (2 μg of DNDs) was added to 1 ml of the Pholiota sp. hot extracts and incubated for 15 min at room temperature. The mixture was slowly supplemented with ammonium sulfate solution the final concentration of 110 mg/ml and centrifuged at 16000 g for 20 min at 10oС. The supernatant obtained was used to determine the amount of non-adsorbed hispidin.
Experimental
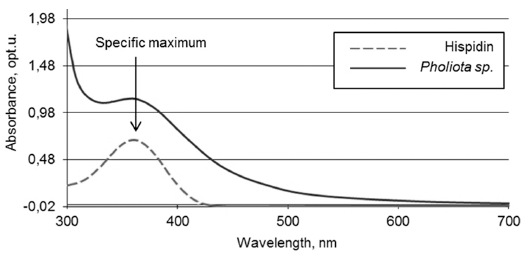
- The presence of hispidin in Pholiota sp. was confirmed by comparing fluorescence and absorbance spectra of aqueous solutions of hispidin and the fungal extract. As seen from Fig. 1-2, both compounds display similar maximum of fluorescence and absorbance at 525 and 357 nm, correspondingly.
- DND-hispidin conjugates
- The results of adsorption experiments presented in Fig. 3 display a high affinity of DNDs towards the synthetic hispidin. Judging by the difference between the light emission intensities measured on the initial and DNDs treated samples, about 75% and 85% of hispidin molecules was adsorbed on DNDs from the hispidin solution when 200 and 400 μl of 3 wt% DND suspensions, respectively, were used.
- Adsorption experiments with the Pholiota sp. hot extracts were also revealed a high binding capacity of DNDs to natural hispidin. After treatment with DNDs, the luminescence of hot extracts was decrease by 65-70% implying a significant amount of hispidin molecules were bound to the surface of DND particles. In these adsorption tests the volume of extracts and mass of S and L fractions were identical. Interesting is the fact that the adsorption of natural hispidin on DNDs does not depend on the particle scale, since the both fractions displayed similar adsorption capacity although the values of their average diameters differ by more than seven times. It contradicts the well-known “size effect” observed in a number of studies on adsorption of various substances on nanodiamonds [22].
- The desorption experiments were carried out on DND conjugates with the synthetic and natural hispidin using the phosphate buffer, NaCl and CaCl2 as an eluent. It was found that neither of the reagents allows a meaningful release of the adsorbed hispidin molecules (both type of hispidin – synthetic and natural) from the DND surface. To check the possibility of pH mediated desorption, the titration experiments were conducted using 1 M HCl, and 1 M NaOH solutions to adjust the pH of the eluent solutions to 2, 3 and 9 values. It was observed that applying the alkaline solution did not lead to desorption of hispidin. The use of solutions with low pH resulted in release from DNDs a relatively small amount of the adsorbed synthetic hispidin molecules (about 0.2%).
- DND-BSA-hispidin conjugates
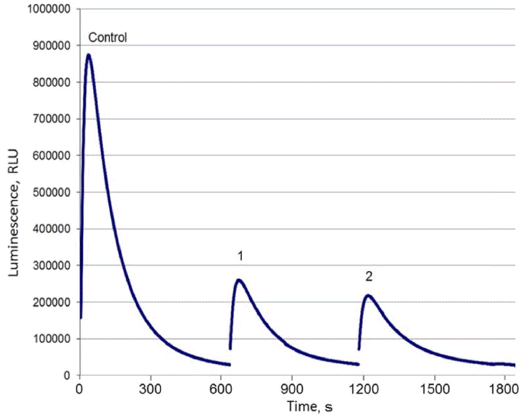
- Adsorption properties of DND-BSA complexes were found to be similar to those of the bared DND particles. As shown in Fig. 4, the luminescence of Pholiota sp. hot extract after treatment with DND-BSA complexes amounts to about 23% of that recorded on the control sample. Thus, more than 77% of natural hispidin molecules contained in the initial extract was bound by DND-BSA complexes.
- However, desorption behavior of hispidin attached to DND-BSA complexes is noticeably different compared to that observed on DND-hispidin conjugates. Fig. 4 shows the luminescence intensity of supernatants which were collected after successive treatment of the obtained DND-BSA-hispidin conjugates with different eluents: two times with 2 M phosphate buffer (pH 7.0) followed with 4 M NaCl solution. Each time the residue was resuspended in the eluent, stirred with shaker for 5 min and centrifuged at 16000 g for 10 min at 10oС. The supernatants thus obtained were tested to determine the eluted hispidin.
- As seen from the Fig. 4, the use of phosphate buffer resulted in elution of hispidin. The first desorption run released about 13% of the adsorbate. The second treatment of DND-BSA-hispidin conjugates with the same eluent also removed adsorbed hispidin from DNDs but with fewer yield. The total amount of the eluated hispidin due to successive desorption in phosphate buffer accounts for 21%. Addition of NaCl did not lead to desorption of detectable amount of hispidin.
- As has been shown above, hispidin displays a high affinity to DNDs and forms strong conjugates with the particles. This can explain why only a relatively small fraction of adsorbed hispidin molecules was released from DND-BSA complexes in desorption experiments. We assume that upon mixing DND-BSA complexes with hispidin, available binding centers on the DND surface are first occupied with hispidin molecules and thus not involved later on in the adsorption process. The remainder part of hispidin molecules are absorbed on BSA and can be then desorbed with corresponding eluent. Thus, the use of previously treated DND-BSA complexes with already occupied (at least partially) adsorption sites on DNDs can result in an enhanced desorption of the bound hispidin. To check the assumption, the DND-BSA complexes, which have been used in the experiments described above, were treated with the fungal extracts following the same procedure (adsorption, then desorption with the phosphate buffer). The results on adsorption and desorption with the previously treated DND-BSA complexes are presented on Fig. 5. About 66% of natural hispidin molecules contained in the initial extract was absorbed by DND-BSA complexes from which more than 37 % were desorbed.
Results and discussion
Fig. 3
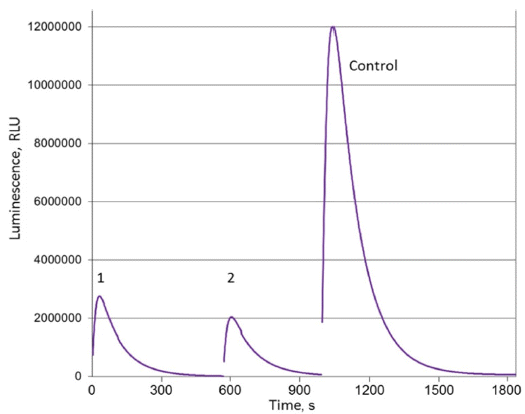
Luminescence of supernatants obtained after adsorption experiments with DNDs and synthetic hispidin: 1 - 200 μl of DND suspension, 2 - 400 μl of DND suspension.

Fig. 4
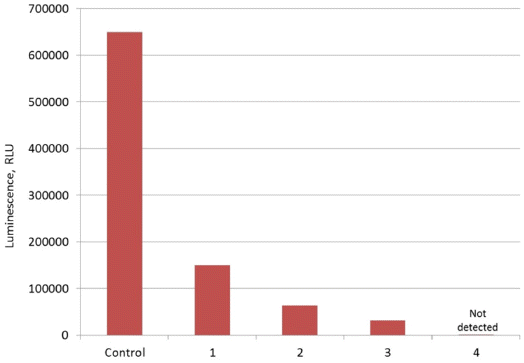
Maximum luminescence measured in experiments with DND-BSA complexes and natural hispidin: Control – initial hot extract, 1 – extract after treatment with DNDBSA complexes, 2 – supernatant after first treatment of DND-BSA-hispidin conjugates with phosphate buffer, 3 – supernatant after second treatment of DND-BSA-hispidin conjugates with phosphate buffer, 4 – supernatant after treatment of DND-BSA-hispidin conjugates with NaCl solution.

- The interaction of detonation nanodiamonds with synthetic and natural hispidin containing in extracts from Pholiota sp. fungus was investigated in this study. The bioluminescence method based on the use of in vitro luminescent system, which was isolated from the fungus Armillaria borealis, was applied to detect the hispidin content. Adsorption experiments revealed a high affinity of hispidin to DND particles. It was found that the hispi-din molecules were strongly attached to the DND surface and the use of a phosphate buffer, NaCl and CaCl2 solutions, as well as aqueous alkaline and acid solutions did not result in a meaningful release of adsorbed hispidin from DNDs. The mechanisms that enable such strong and irreversible attachment of hispidin to DNDs are unclear and require further investigation. DND-BSA complex, where DNDs serve as a carrier for the protein while the latter acts as a sorbent for the hispidin molecules, was developed and applied in hispidin adsorption/ desorption tests. The use of the phosphate buffer resulted in desorption of hispidin from DND-BSA complexes, while addition of NaCl did not lead to release of the substance. The amount of hispidin eluted in two successive desorptions accounts for 21% of the total adsorbed molecules. The secondary use of the previously treated DNDBSA complexes enhanced desorption of the bound hispidin. The results obtained suggest the application of DNDs as a carrier of hispidin in medical application while DND-BSA complex can be employed for its isolation from fungal extracts.
Conclusions
-
Acknowledgements
- The authors are grateful to Korea Atomic Energy Research Institute (KAERI) for financial support of the research.
Acknowledgement
- 1. V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi: Nat. Nanotechnol., 7 (2012) 11. .ArticlePubMed
- 2. A. Vul and O. Shenderova: Detonation Nanodiamonds. Science and Applications, Pan Stanford, Singapore (2014) 400. .ArticlePubMed
- 3. H. Tinwala and S. Wairkar: Mater. Sci. Eng. C, 97 (2019) 913. .ArticlePubMed
- 4. A. M. Schrand, H. J. Huang, C. Carlson, J. J. Schlager, E. Osawa, S. M. Hussain and L. Dai: J. Phys. Chem. B, 111 (2007) 2. .ArticlePubMed
- 5. A. P. Puzyr, A. E. Burov, V. S. Bondar and Y. N. Trusov: Nanotechnol. Russ., 5 (2010) 137. .Article
- 6. P. C. Soo, C. J. Kung, Y. T. Horng, K. C. Chang, J. J. Lee and W. P. Peng: Anal. Chem., 84 (2012) 7972. .ArticlePubMed
- 7. K. Turcheniuk and V. N. Mochalin: Nanotechnology, 28 (2017) 1. .ArticlePubMed
- 8. M. D. Kalaras, J. P. Richie, A. Calcagnotto and R. B. Beelman: Food Chem., 233 (2017) 429. .ArticlePubMed
- 9. S. Prasad, H. Rathore, S. Sharma and A. S. Yadav: Int. J. Food Sci. Nutr. Diet., 4 (2015) 221. .
- 10. P. Greeshma, K. S. Ravikumar, M. N. Neethu, M. Pandey, K. F. Zuhara and K. F. Janardhanan: Int. J. Med. Mushrooms, 18 (2016) 235. .ArticlePubMed
- 11. J. H. Lim, Y. M. Lee, S. R. Park, D. H. Kim and B. O. Lim: Anticancer Res., 34 (2014) 4087. .
- 12. W. C. Lin, J. S. Deng, S. S. Huang, S. H. Wu, H. Y. Lin and G. J. Huang: RSC Adv., 7 (2017) 7780. .Article
- 13. T. Rahmani, A. Hajian, A. Afkhami and H. Bagheri: New J. Chem., 42 (2018) 7213. .Article
- 14. Q. X. Hu, H. X. Wang and T. B. Ng: Int. J. Med. Mushrooms, 14 (2012) 271. .ArticlePubMed
- 15. C. R. Wang, R. Zhou, T. B. Ng, J. H. Wong, W. T. Qiao and F. Liu: Environ. Toxicol. Pharmacol., 37 (2014) 626. .ArticlePubMed
- 16. Y. Zhang, Z. Liu, T. B. Ng, Z. Chen, W. Qiao and F. Liu: Biochime, 99 (2014) 28. .ArticlePubMed
- 17. J. Velisek and K. Cejpek: Czech J. Food Sci., 29 (2011) 87. .Article
- 18. I. K. Lee, S. M. Cho, S. J. Seok and B. S. Yun: Mycobiology, 36 (2008) 55. .ArticlePubMedPMC
- 19. A. P. Puzyr, S. E. Medvedeva, A. E. Burov, Y. P. Zernov and V. S. Bondar: Dokl Biochem Biophys., 480 (2018) 173. .ArticlePubMed
- 20. K. V. Purtov, V. N. Petushkov, M. S. Baranov, K. S. Mineev, N. S. Rodionova, Z. M. Kaskova, A. S. Tsarkova, A. I. Petunin, V. S. Bondar, E. K. Rodicheva, S. E. Medvedeva, Y. Oba, A. S. Arseniev, S. Lukyanov, J. I. Gitelson and I. V. Yampolsky: Angew. Chem., Int. Ed., 54 (2015) 8124. .ArticlePubMed
- 21. A. P. Puzyr, A. E. Burov and V. S. Bondar: Fuller. Nanotub. Car. N., 23 (2015) 93. .Article
- 22. L. C. L. Huang and H. C. Chang: Langmuir, 20 (2004) 5879. .ArticlePubMed
Figure & Data
References
Citations
Citations to this article as recorded by 

Interaction of Detonation Nanodiamonds with Hispidin
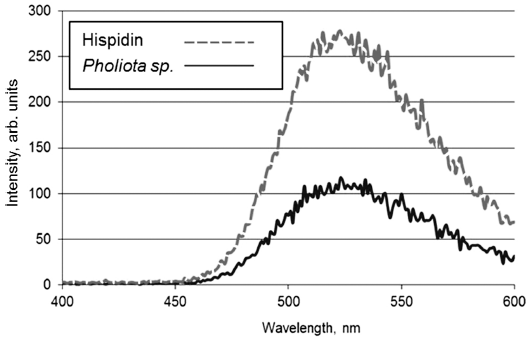


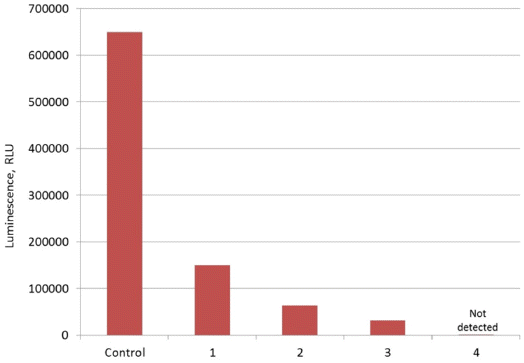
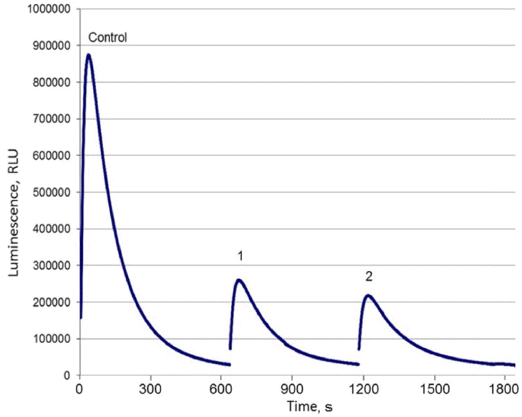
Fig. 1
Fluorescence of synthetic hispidin solution and Pholiota sp. hot extract.
Fig. 2
Absorbance spectra of synthetic hispidin solution and Pholiota sp. hot extract.
Fig. 3
Luminescence of supernatants obtained after adsorption experiments with DNDs and synthetic hispidin: 1 - 200 μl of DND suspension, 2 - 400 μl of DND suspension.
Fig. 4
Maximum luminescence measured in experiments with DND-BSA complexes and natural hispidin: Control – initial hot extract, 1 – extract after treatment with DNDBSA complexes, 2 – supernatant after first treatment of DND-BSA-hispidin conjugates with phosphate buffer, 3 – supernatant after second treatment of DND-BSA-hispidin conjugates with phosphate buffer, 4 – supernatant after treatment of DND-BSA-hispidin conjugates with NaCl solution.
Fig. 5
Luminescence measured in experiments with the previously treated DND-BSA complexes and natural hispidin: Control – initial hot extract, 1 – supernatant after adsorption, 2 – supernatant after treatment of DND-BSA-hispidin conjugates with phosphate buffer.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Interaction of Detonation Nanodiamonds with Hispidin
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article





