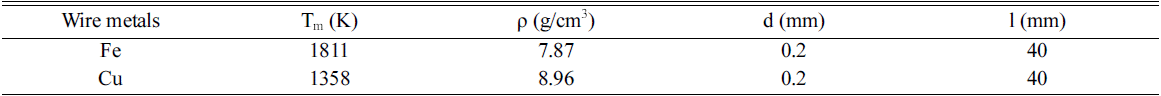Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 27(6); 2020 > Article
-
ARTICLE
- Fabrication and Characterization of Immiscible Fe-Cu Alloys using Electrical Explosion of Wire in Liquid
- Chu Dac Phuca, Nguyen Minh Thuyetb, Jin-Chun Kima,*
-
Journal of Korean Powder Metallurgy Institute 2020;27(6):449-457.
DOI: https://doi.org/10.4150/KPMI.2020.27.6.449
Published online: November 30, 2020
a School of Materials Science and Engineering, University of Ulsan, 12, Techno saneop-ro 55beon-gil, Nam-gu, Ulsan, 44776, Republic of Korea
b School of Materials Science and Engineering, Hanoi University of Science and Technology, No 1, Dai Co Viet, Hai Ba Trung,Hanoi 100000, Vietnam
- *Corresponding Author: Jin-Chun Kim, TEL: +82-52-712-8061, FAX: +82-52-259-1688, E-mail: jckimpml@ulsan.ac.kr
- - Chu Dac Phuc: 학생, Nguyen Minh Thuyet: 전임강사, 김진천: 교수
• Received: October 11, 2020 • Revised: November 10, 2020 • Accepted: November 10, 2020
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,159 Views
- 11 Download
- 3 Crossref
Abstract
- Iron and copper are practically immiscible in the equilibrium state, even though their atomic radii are similar. As non-equilibrium solid solutions, the metastable Fe-Cu alloys can be synthesized using special methods, such as rapid quenching, vapor deposition, sputtering, ion-beam mixing, and mechanical alloying. The complexity of these methods (multiple steps, low productivity, high cost, and non-eco-friendliness) is a hinderance for their industrial applications. Electrical explosion of wire (EEW) is a well-known and effective method for the synthesis of metallic and alloy nanoparticles, and fabrication using the EEW is a simple and economic process. Therefore, it can be potentially employed to circumvent this problem. In this work, we propose the synthesis of Fe-Cu nanoparticles using EEW in a suitable solution. The powder shape, size distribution, and alloying state are analyzed and discussed according to the conditions of the EEW.
- Nanomaterials are gradually becoming favored materials for many researchers. With very small size, under 100 nm, nanomaterials have the properties that cannot be achieved by normal size materials. Among those properties, the most remarkable features of nanomaterials are their unique physical and chemical properties. Nanoparticle is a branch of nanomaterials, with all three external dimensions in the nanoscale range. Despite of being only a particle, these nanosized particles act like a complete unit. If the nanoparticle is made of a single metal, it is called monometallic nanoparticle. Bimetallic nanoparticles are nanoparticles that included two different metal elements on them. Bimetallic nanoparticles can be considered as an advanced alloy based on nanoparticles from two base metals. Because it is a nanoparticle, BNP will have unique nano properties as other nanoparticles. Also, we can study the promise new properties from combining two metals.
- Iron and its alloys are used in many aspects of life including magnetic materials. However, when being alloyed, the magnetism of iron decreases quickly, limiting its application. However, the Fe-Cu alloy is very special, it maintains the magnetism of iron in the alloy even with a large amount of Cu in the alloy. Therefore, the Fe- Cu alloys have become a prospect for creating magnetic iron alloy materials. That unique magnetic properties, accompanied by its high strength and attractive electric and thermal properties make Fe-Cu alloys system become very attractive for scientific researchers [1-5]. In term of Fe-Cu alloys, bimetallic particles and nanoparticles consisting Fe and Cu components have been used in environmental and medical applications thanks to the high removal capacities for pollutant removal, excellent antibacterial and corrosive resistance properties [6]. Due to the fact that the Fe-Cu system is nearly immiscible in equilibrium at room temperature and up to 600°C [7], the fabrication of this material has the difficulties in processing. However, the usefulness in this material’s application still attracts attention of researchers and manufactures, therefore, several techniques have been studied and applied to synthesis Fe-Cu alloys included rapid quenching [8], vapor deposition [9, 10], sputtering [11], ionbeam mixing [12], mechanical alloying [13, 14], and high-pressure torsion [15]. Thus, developing a new method for synthesizing nanostructured Fe–Cu alloys is a critical challenge for all researcher. From an economics viewpoint, proposing a possible route which can simply manufacture nanostructure Fe–Cu alloys, the ones with low cost but still good quality is needed.
- The electrical explosion of wire (EEW) is a process of explosive destruction of metal wires under the action of great density current (> 1010 A/m2) [16]. This process is accompanied by scattering products, shock waves and electromagnetic radiation. EEW is characterized by the following peculiarities: time of explosion is 10-5~10-8 s; temperature at the moment of explosion can reach the value more than 104 K; pressure ~109 Pa; velocity of product recession is from 1000 to 5000 m/s [17]. This technique has attracted attention for fabrication of various metallic nanopowders due to the simple and low-cost processing [18]. EEW can be used to cheaply and efficiently produce nanoparticles at a rate of 50-300 grams per hour and at a purity of above 99%. The particles can be as small as 10 nm but are most commonly below 100 nm in diameter. This is a simply physical process and a suitable top-down approach for nanosized powder production. Material of the wire transmutes into the nanoscale particles in accordance with certain conditions. Extremely nonequilibrium condition of the EEW cause some unusual properties of nanopowders. They are steady against oxidation and sintering at room temperature and characterized with high diffusion activity at the heating [13].
- Herein, the Fe-Cu bimetallic nanoparticles were synthesized by using EEW for twisted Fe and Cu wires in ethanol. The composition of the alloy could be change simply by changing the numbers or wires twisted with each metal. We also synthesized iron, copper and their mixed nanopowders for comparison purposes.
Introduction
- Materials
- Iron wires (purity of 99%) with diameter of 0.2 mm and copper wires (purity of 99%) with diameter of 0.2 mm were used. Ethanol was used as solution for the explosions. All materials were commercially available and used as the received materials without further purification.
- Experiment procedure
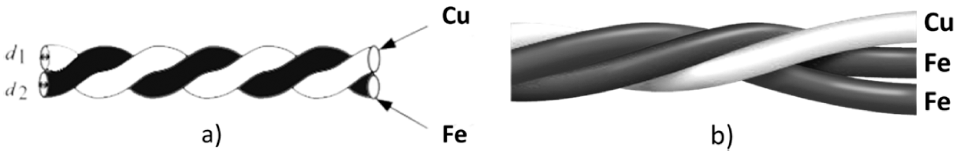
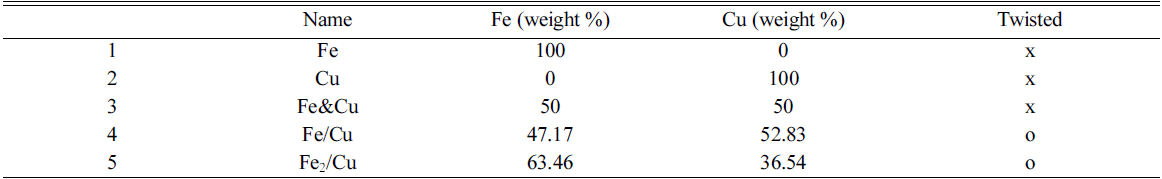
- The iron and copper nanoparticles were prepared by exploding 0.2 mm ø iron wires (99% pure) and 0.2 mm ø copper wires (99% pure) in ethanol, respectively. These two powders also be taken to mix together in one solution to get Fe&Cu mixed nanoparticles. The explosion progress was established continuously and alternately between iron and copper wires to achieve the finest mixing state. In other experiment, Fe wires (0.2 mm ø) and Cu wires (0.2 mm ø) were twisted together (with the ratio 1:1 and 2:1) in order to synthesis Fe/Cu and Fe2/Cu nanoparticles (Fig. 1). The composition content of the metals in the twisted wire was calculated by the specific density and the diameters of the wires (Table 1). They are all iron and copper nanoparticles but been prepared by different method to compare and emphasize the important of twisted and explode two metal wires together in order to get desired nanoparticles.
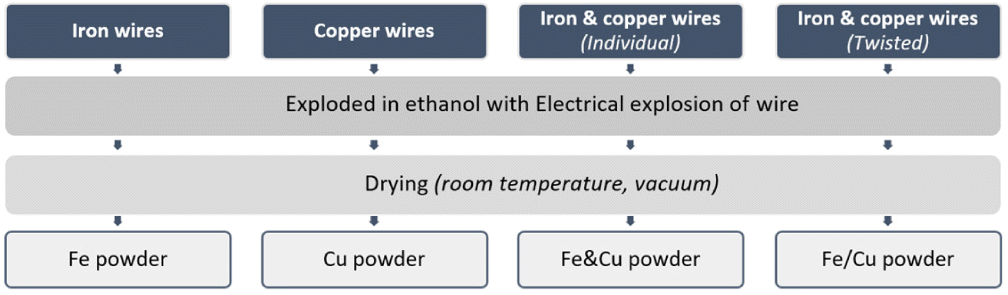
- Wires were cleaned with ethanol to remove surface contaminants before experiments. The wire characteristics are given in Table 2 (Tm is the metal melting temperature, ρ is the specific metal density, d is the wire diameter, l is the wire length).
- The schematic steps of the experiment procedure were presented in Fig. 2. All the experiments were conducted in the EEW machine with an ethanol solution bath at room temperature condition (Fig. 3). The applied voltage and the capacitance of the exploding circuit across the 4- mm-long wire was 3.42 kV and 30 μF, respectively. After the explosion, the powders were collected from solutions by drying at room temperature under vacuum condition for 24 hours.
- In this experiment, 1 iron wire with 1 copper wire was exploded each time, so the productivity of the process can be calculated with this equation:
- Similar, with Fe2/Cu experiment, we have this equation:
- Here, m is weight of wire (calculated with density and wire dimension); t is explosion time each (30 seconds). So, the productivity rate can be calculated as 2.5 to 3.8 g/ hour, depend on how many metal wires were exploded at the same time.
- Characterization Method
- Phase characterizations of the prepared nanopowders were observed by using the X-ray diffractometer (XRD, Ultima IV, Rigaku). The XRD pattern was performed from 20 to 100° (2θ) with Cu Kα radiation (λ = 1.5406 Å). The field emission scanning electron microscope (FE-SEM, JSM-6500, JEOL Ltd) equipped with energydispersive spectroscopy (EDS), transmission electron microscope (TEM - H-8100, Hitachi) were also used to determine the particle morphology and to confirm the size of the particles.
Experiment details
- Morphology Analysis
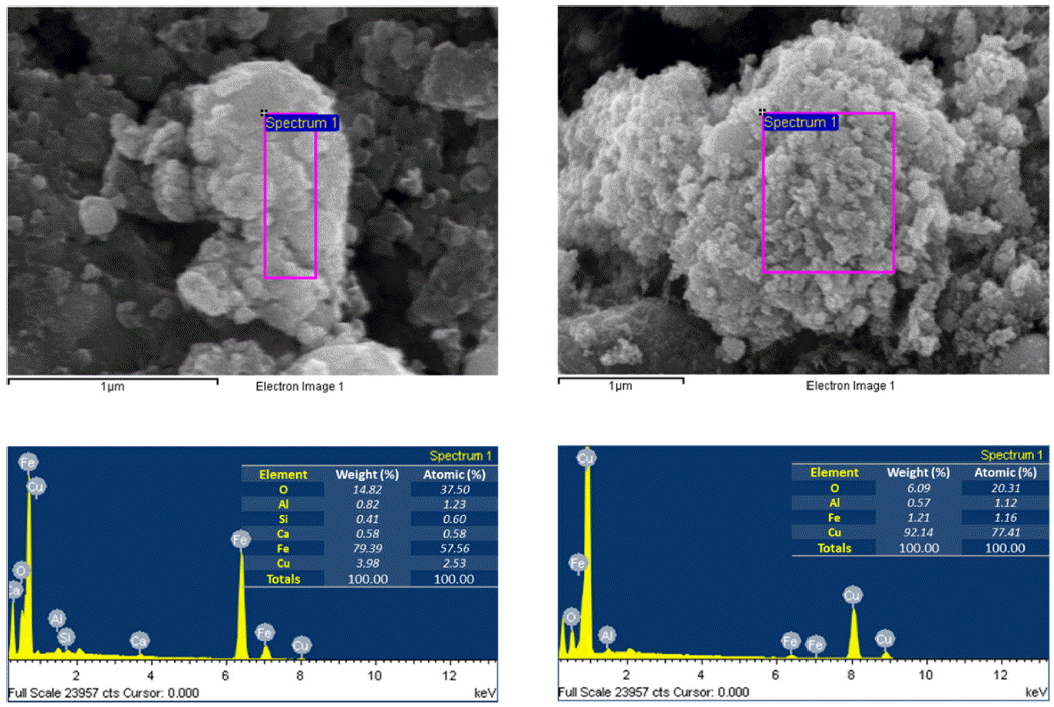
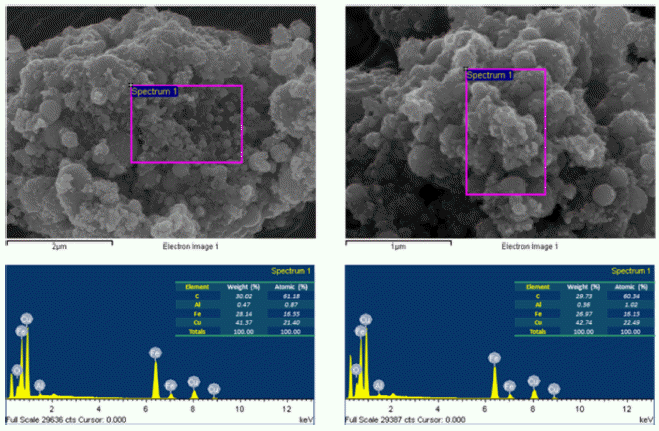
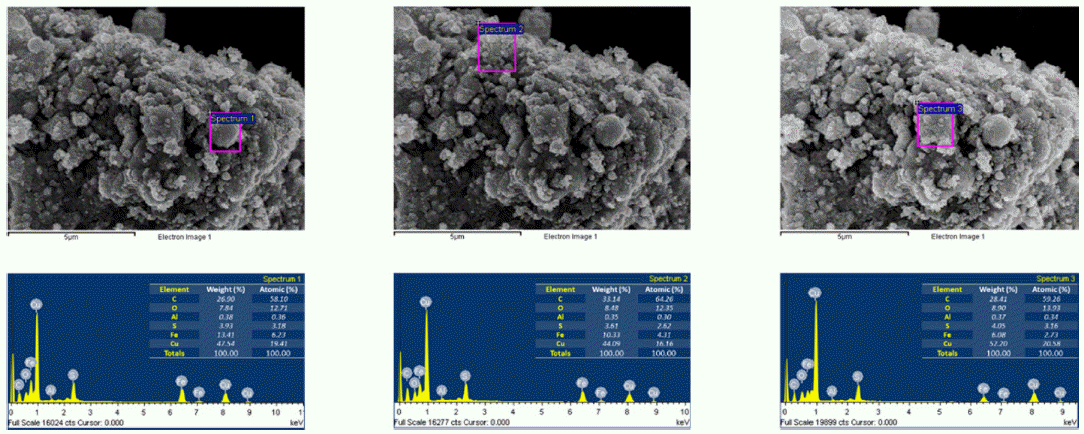
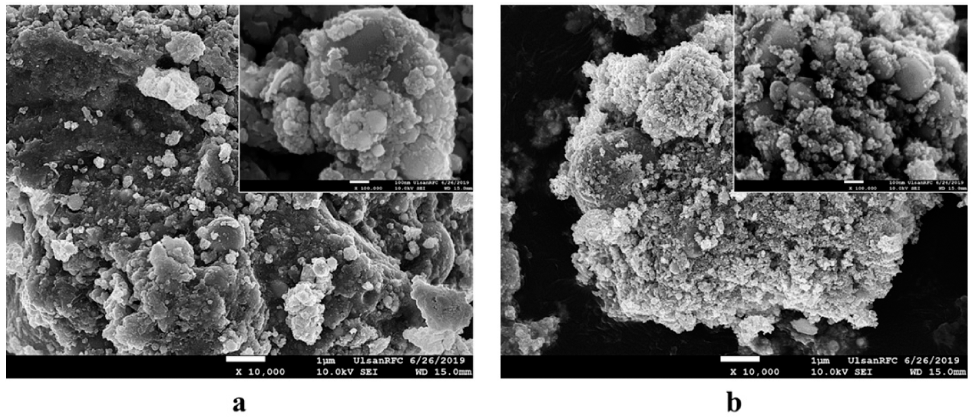
- The morphology and size of as-prepared powders produced by EEW were investigated by FE-SEM. Spherical nanoparticles were initially observed for each sample. With a same condition, Fe particles have bigger size than Cu particles, their particle size distribution range from 50-200 nm and 10-100 nm, respectively (Fig. 4). In other experiment, as we can observed in Fig. 5, Fe and Cu mixed nanopowders have similar shape and size with Cuonly nanopowders. The particle size could be classified into two groups based on diameter as nanoparticles (with the diameters under 100 nm) and fine particles (with the diameters slightly larger than 100 nm). This powder is the mixture of Fe and Cu powder, so we can know that some scattered particles (Fe) were covered by many smaller particles (Cu). When twisted wire together, both Fe2/Cu particles (Fig. 6.a) and Fe/Cu particles (Fig. 6.b) have spherical shape and particle size spread from 50 to 120 nm. Different to Fe&Cu mixed powder, the size of metal particles was not clearly split into two groups but spread evenly. Fig. 7 show the EDS images of iron and copper exploded in ethanol. As we can observe, there is a significant amount of oxygen in both samples, beside the original metal. The existence of oxygen can be explained by the formation of metal oxides during the experiment process. After the explosions, iron and copper nanoparticles reacted with oxygen to form the oxide film, covering the nanoparticles. In Fig. 8 and Fig. 9, EDS images of Fe2/Cu nanoparticles and Fe/Cu nanoparticles can be observed. We can notice the existence of a significant amount of carbon element in both samples. This amount of carbon come from the ethanol used for experimental solution. Because there is no reaction between carbon and metal in this experiment, the carbon exists in the form of layers covering the metal particles.
- Phase Analysis
- For determination of the phase structure of the exploded products the XRD analysis was conducted. Fig. 10 shows the XRD pattern of the as-prepared powders. According to the result, the XRD pattern of the synthesized Fe powders shows a dominant peak at 2θ = 44,58° which corresponds to the (110) plane of the face centered cubic (fcc) with a little percentage of iron oxide (Fe2O3) that could be observed by small peaks at 2θ = 64,98° (110); and 82.49° (111)[19]. With as-exploded Cu powder, the XRD pattern shows mainly the peaks are in good conformity with the pure cubic copper phase with the peak positions at 2θ = 43.24° (111); 50.36° (200); 74.04° (220); and 89.86° (311). However, the other peaks corresponding to the presence of Cu2O phase (at 2θ = 36.36° (111); 42.30° (200); and 61.32° (220)) were also observed [20]. The appearance of iron oxide and copper oxide phase in the as-exploded powders is attributed to the interaction of Fe and Cu with oxygen diluting in the solvent during exploding stage.
- According to XRD peak analyses of synthesized Fe/Cu powders, the major reflections have been attributed to copper and iron, however, the peak of Fe were weakened by copper implantation. The other reflections corresponding to copper oxide and iron oxide also observed. In addition, it is found that the new peaks belong to new phases of Cu bearing Fe0.1Cu0.9, Fe0.2Cu0.8, Fe0.6Cu0.4 and Fe4Cu3 were examined. The phases of Fe0.2Cu0.8 can be clearly observed the three well-defined peaks at 2θ = 43.26°, 50.38°, 74.04°. The other peaks at 2θ = 89.82° and 2θ = 95.06° is attributed to the presence of the phase of Fe0.1 Cu0.9. Also, there are other small peaks at 2θ = 44.68° and 2θ = 46.02°, correspond to the phases of Fe4-Cu3 and Fe0.6Cu0.4, respectively [21, 22]. This result demonstrates that the bimetallic Fe/Cu particles were synthesized successfully by explosion of the initial twisted wires in the ethanol condition.
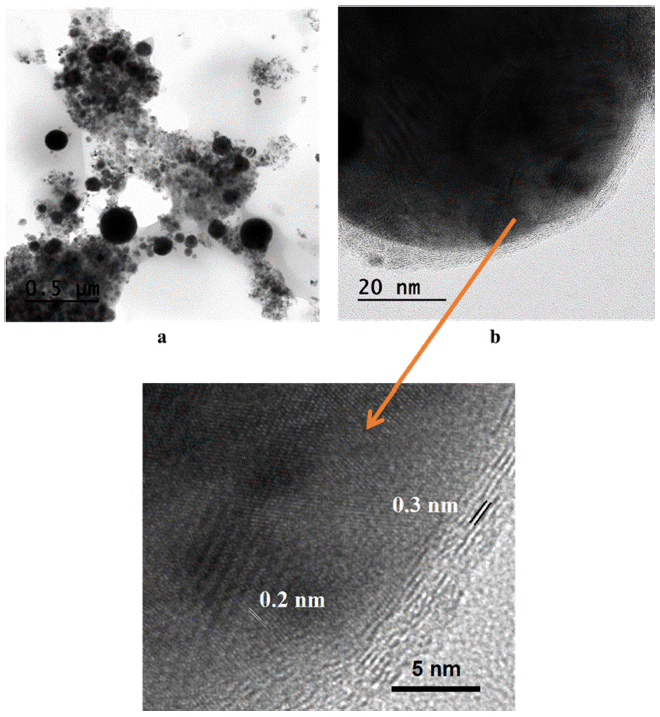
- The morphology and shape of the synthesized Fe/Cu nanopowders were observed by TEM analysis, the results were presented in Fig. 11 and Fig. 12. It could be seen that the as-prepared powders have a spherical form with the particles size was extensively spread from 10 to 200 nm. According to the TEM images, the particles size consists of big and small particles with a tendency increasing in the amount of the small one with the higher of Fe content in the twisted wires. This is attributed to the difference in the melting point and the electrical conductivity between Fe and Cu. The TEM images also reveals that there is a layer of material covered the particles after exploding which is confirmed graphitic carbon layer with the thickness is about 0.3 nm. The presence of the graphitic layer is attributed to the existence of the large amount of carbon element diluted in the ethanol solvent [23]. The TEM results further supports the XRD analysis regarding the formation of bimetallic Fe/Cu particles. Thus, the high-resolution TEM images depict the lattice fringes of the phases as have observed through XRD pattern with the lattice spacing d = 0.2 nm which is the same with the lattice spacing of Cu and Fe.
Results and discussion
Fig. 4
FE-SEM images of (a) iron and (b) copper nanoparticles prepared by EEW at low and high magnification.

Fig. 5

FE-SEM images of Fe&Cu mixed nanoparticles prepared by EEW at (a) low and (b) high magnification.

Fig. 6

FE-SEM images of (a) Fe2/Cu and (b) Fe/Cu nanoparticles prepared by EEW at low and high magnification.

Fig. 10
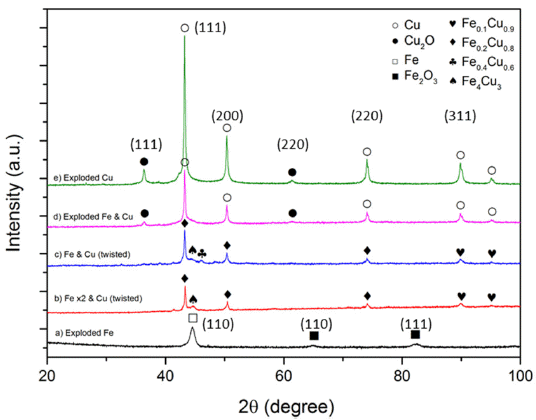
XRD patterns of exploded Iron, exploded Copper, exploded Iron and Copper, exploded Iron and Copper (twisted).

- In summary, the present work has successfully fabricated the Fe/Cu nanopowders by using explosion of twisted wires in ethanol. Control factors were electrical parameters, characteristics of wires, and surrounding ambiences. Despite their little mutual solubility in the solid state, the nanoparticles were prepared and their characteristics were investigated. The nanopowders were nearly spherical shape with the mean particle size distribution range from 10-200 nm. According to the XRD analysis results, the fabricated nanoparticles consist of Fe, Cu and phases of Fe0.1Cu0.9, Fe0.2Cu0.8, Fe0.4Cu0.6, and Fe4Cu3. In addition, the as-synthesized nanoparticles were covered by a very thin graphitic carbon layer due to the existence of carbon element in the ethanol. This work showed a simple and promising method to synthesize the metastable Fe/Cu alloys. The obtained nano-sized particles can be a promising material to be applied widely in environmental and medical applications.
Conclusion
- 1. M. Hasebe and T. Nishizawa: Calphad, 5 (1981) 105. .Article
- 2. X. Huang and T. Mashimo: J. Alloys Compd., 288 (1999) 299. .Article
- 3. A. W. Weeber: J. Phys. F. Met. Phys., 17 (1987) 809. .Article
- 4. J. He, J. Z. Zhao and L. Ratke: Acta Mater., 54 (2006) 1749. .Article
- 5. G. Mazzone and M. V. Antisari: Phys. Rev. B, 54 (1996) 441. .ArticlePubMed
- 6. O. V. Bakina, E. A. Glazkova, N. V. Svarovskaya, N. G. Rodkevich and M. I. Lerner: Mater. Lett., 242 (2019) 187. 9.Article
- 7. A. R. Yavari, P. J. Desré and T. Benameur: Phys. Rev. Lett., 68 (1992) 2235. .ArticlePubMed
- 8. W. Klement: Trans. Metall. Soc. AIME, 233 (1965) 1180. 9.
- 9. E. F. Kneller: J. Appl. Phys., 35 (1964) 2210. .Article
- 10. C. L. Chien, S. H. Liou, D. Kofalt, W. Yu, T. Egami, T. J. Watson and T. R. McGuire: Phys. Rev. B, 33 (1986) 3247. .ArticlePubMed
- 11. K. Sumiyama and Y. Nakamura: Rapidly Quenched Metals, S. Steeb and H. Warlimont (Ed.), Elsveier, (1985) 859. .Article
- 12. L. J. Huang and B. X. Liu: Appl. Phys. Lett., 57 (1990) 1401. .Article
- 13. K. Uenishi, K. F. Kobayashi, S. Nasu, H. Hatano, K. N. Ishihara and P. H. Shingu: Z. Metallked., 83 (1992) 132. .
- 14. A. R. Yavari and P. J. Desré: Ordering and Disordering in Alloys, A. R. Yavari (Ed.), Elsevier, (1992) 414. .Article
- 15. A. Bachmaier, M. Kerber, D. Setman and R. Pippan: Acta Mater., 60 (2012) 860. .ArticlePubMedPMC
- 16. V. A. Burtshev, N. V. Kalinin and A. V. Luchinskii: Electrical Explosion of Wires and Its Use in Electro-Physical Equipment, Energoatomizdat, Moscow, 1980. .
- 17. O. Nazarenko: Proceedings of European Congress of Chemical Engineering (ECCE-6), (2007). .
- 18. Y.-S. Kwon, J.-C. Kim, A. P. Ilyin, O. B. Nazarenko and D. V. Tikhonov: J. Korean Powder Metall. Inst., 19 (2012) 40. .Article
- 19. M. Rengasamy, K. Anbalagan, S. Kodhaiyolii and V. Pugalenthi: RSC Adv., 6 (2016) 9261. .Article
- 20. K. -P. Jeong, S. -W. Yang and J. -G. Kim: Arch. Metall. Mater., 63 (2018),1449. .
- 21. Z. G. Dan, H. Ni, B. F. Xu, J. Xiong and P. Y. Xiong: Thin Solid Films, 492 (2005) 93. .Article
- 22. E. Çelik, S. Islak and C. İlkılıç: Mater. Tehnol., 48 (2014) 881. .
- 23. N. M. Thuyet and J. C. Kim: Arch. Metall. Mater., 64 (2019) 893. .
Figure & Data
References
Citations
Citations to this article as recorded by 

- Identification of the reconstruction induced high-entropy spinel oxide nanosheets for boosting alkaline water oxygen evolution
Xuexue Wang, Runqing Lu, Shanhe Gong, Shaokang Yang, Wenbo Wang, Zhongti Sun, Xiaozhen Zhang, Jun Liu, Xiaomeng Lv
Chemical Engineering Journal.2025; 503: 158488. CrossRef - Trends in bimetallic nanomaterials and methods for the removal of p-nitrophenol and its derivatives from wastewater
M. S. Qatan, F. Arshad, M. Miskam, G. A. Naikoo
International Journal of Environmental Science and Technology.2024; 21(5): 5247. CrossRef - Control of cluster coalescence during formation of bimetallic nanoparticles and nanoalloys obtained via electric explosion of two wires
K.V. Suliz, A.Yu. Kolosov, V.S. Myasnichenko, N.I. Nepsha, N.Yu. Sdobnyakov, A.V. Pervikov
Advanced Powder Technology.2022; 33(3): 103518. CrossRef
-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- Fabrication and Pore Characteristics of Metal Powder Filters with a Cross-Sealed Honeycomb Shape Using Material Extrusion Additive Manufacturing
- Fabrication and Characterization of NiCo2O4/Ni Foam Electrode for Oxygen Evolution Reaction in Alkaline Water Splitting
- Fabrication and Characteristics of YSZ-TiC Ceramics Composite by Using Hot Pressing
Fabrication and Characterization of Immiscible Fe-Cu Alloys using Electrical Explosion of Wire in Liquid


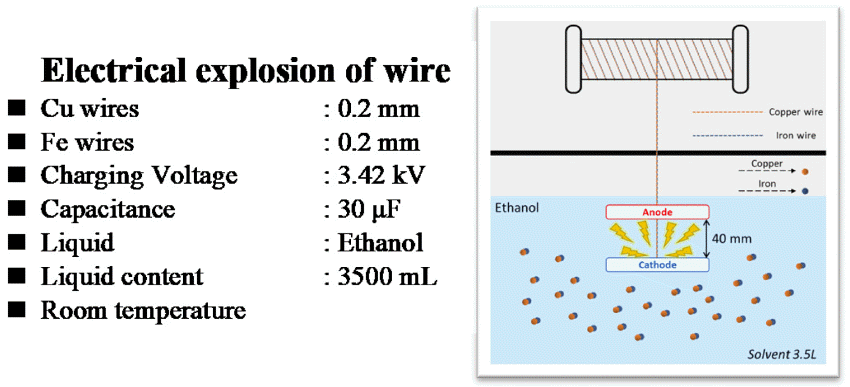
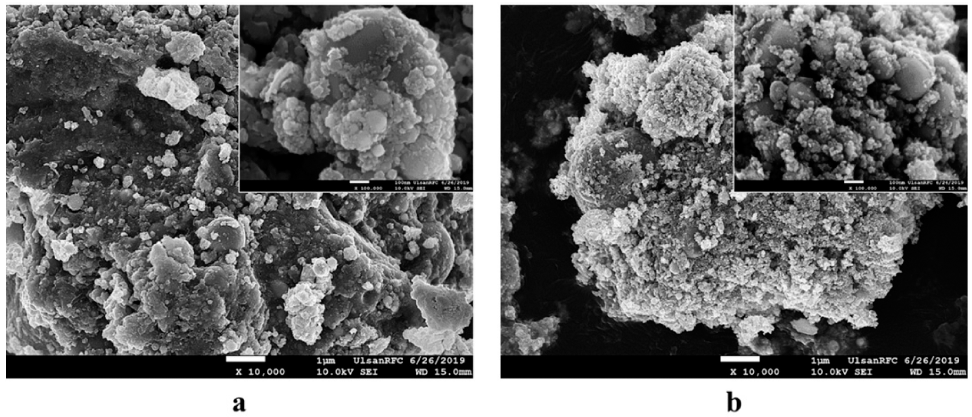


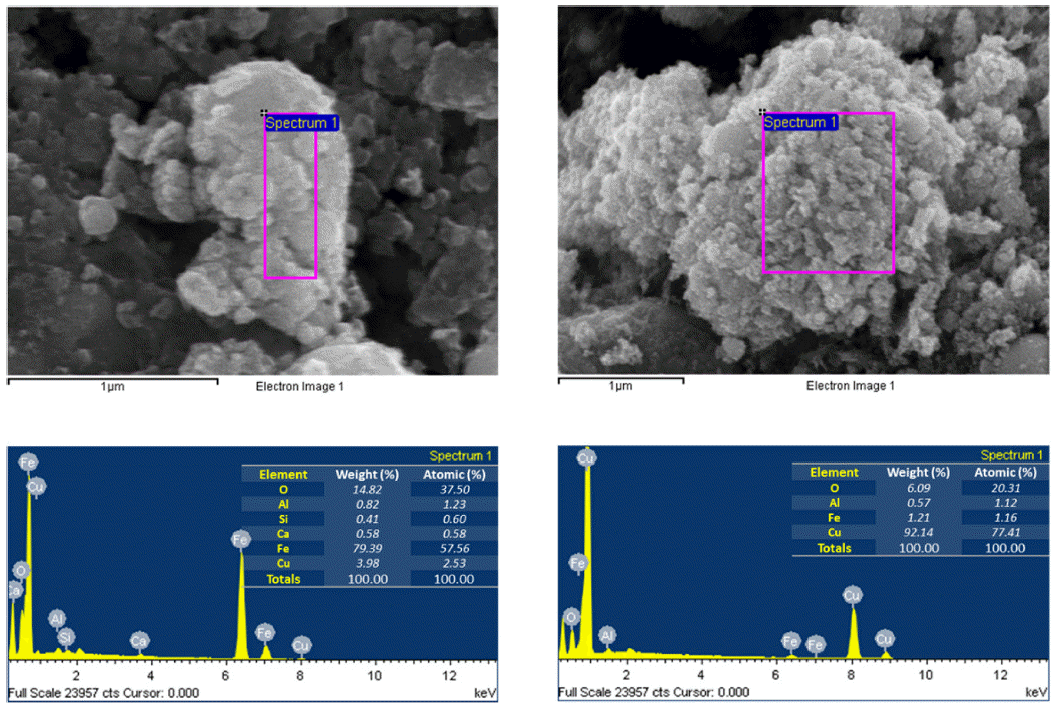
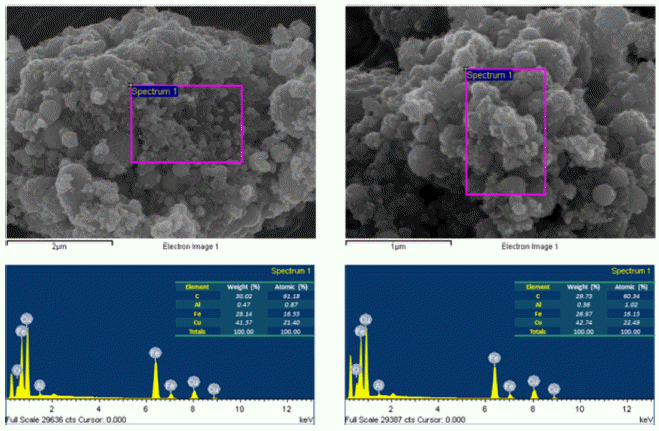
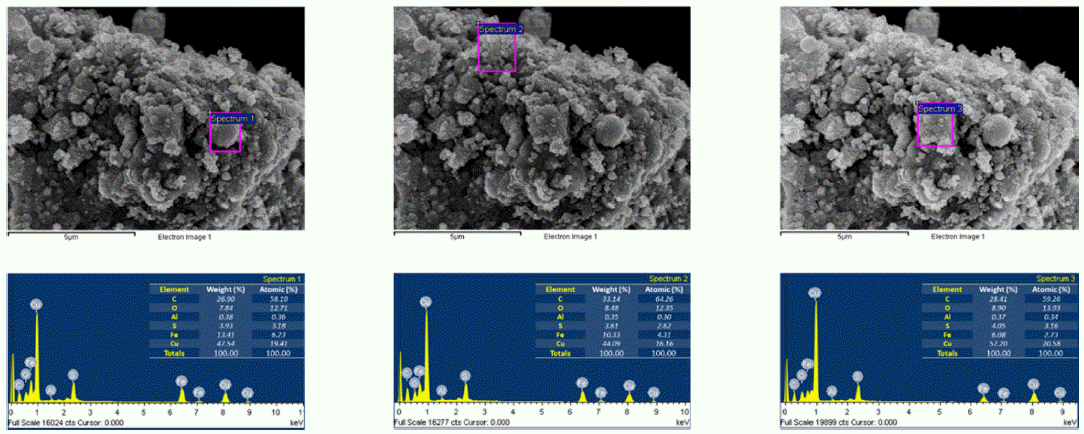

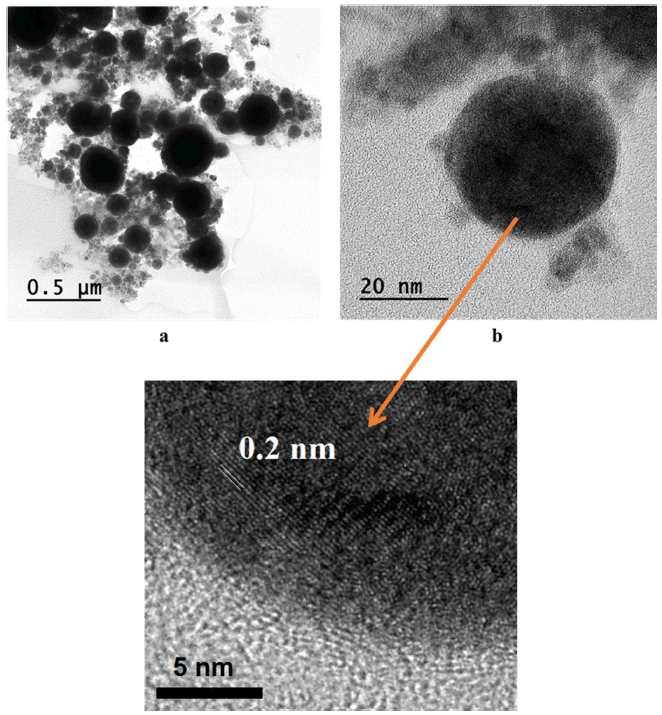
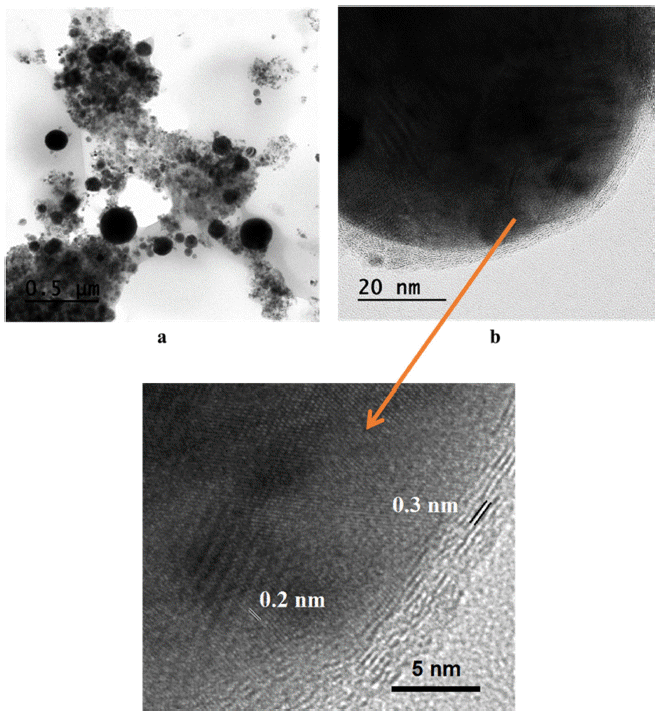
Fig. 1
Copper and Iron wires were twisted together (a) Fe/Cu (1-1) (b) Fe2/Cu (2-1).
Fig. 2
Schematic diagram showing experimental steps for processing.
Fig. 3
Conditions used for the electrical explosion of wire process.
Fig. 4
FE-SEM images of (a) iron and (b) copper nanoparticles prepared by EEW at low and high magnification.
Fig. 5
FE-SEM images of Fe&Cu mixed nanoparticles prepared by EEW at (a) low and (b) high magnification.
Fig. 6
FE-SEM images of (a) Fe2/Cu and (b) Fe/Cu nanoparticles prepared by EEW at low and high magnification.
Fig. 7
EDS images of iron and copper nanoparticles prepared by EEW.
Fig. 8
EDS images of Fe2/Cu nanoparticles prepared by EEW.
Fig. 9
EDS images of Fe/Cu nanoparticles prepared by EEW.
Fig. 10
XRD patterns of exploded Iron, exploded Copper, exploded Iron and Copper, exploded Iron and Copper (twisted).
Fig. 11
TEM images of Fe2/Cu nanoparticles prepared by EEW at (a) low and (b) high magnification.
Fig. 12
TEM images of Fe/Cu nanoparticles prepared by EEW at (a) low and (b) high magnification.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Fig. 8
Fig. 9
Fig. 10
Fig. 11
Fig. 12
Fabrication and Characterization of Immiscible Fe-Cu Alloys using Electrical Explosion of Wire in Liquid
Table 1
Metal wire composition
Table 2
Metal wire characteristics
Table 1
Table 2
TOP
 KPMI
KPMI