Articles
- Page Path
- HOME > J Powder Mater > Volume 31(2); 2024 > Article
-
Research Article
- The Effect of TiO2 Addition on Low-temperature Sintering Behaviors in a SnO2-CoO-CuO System
- Jae-Sang Lee, Kyung-Sik Oh, Yeong-Kyeun Paek*
-
Journal of Powder Materials 2024;31(2):146-151.
DOI: https://doi.org/10.4150/jpm.2024.00024
Published online: April 30, 2024
School of Advanced Materials and Electrical Engineering, Andong National University, Andong Kyungbuk, 36729, Republic of Korea
- *Corresponding author: Yeong-Kyeun Paerk, TEL: +82-54-820-5754, E-mail: ykpaek@anu.ac.kr
- J.-S. Lee: Student, K.-S. Oh, Y.-K. Paek: Professor
• Received: March 21, 2024 • Revised: April 17, 2024 • Accepted: April 18, 2024
© The Korean Powder Metallurgy & Materials Institute
- 1,179 Views
- 18 Download
Abstract
- Pure SnO2 has proven very difficult to densify. This poor densification can be useful for the fabrication of SnO2 with a porous microstructure, which is used in electronic devices such as gas sensors. Most electronic devices based on SnO2 have a porous microstructure, with a porosity of > 40%. In pure SnO2, a high sintering temperature of approximately 1300°C is required to obtain > 40% porosity. In an attempt to reduce the required sintering temperature, the present study investigated the low-temperature sinterability of a current system. With the addition of TiO2, the compositions of the samples were Sn1-xTixO2-CoO(0.3wt%)-CuO(2wt%) in the range of x ≤ 0.04. Compared to the samples without added TiO2, densification was shown to be improved when the samples were sintered at 950°C. The dominant mass transport mechanism appears to be grain-boundary diffusion during heat treatment at 950°C.
- Pure SnO2 is an N-type semiconductor (Eg ~3.6eV) with a tetragonal structure of rutile phase. The chemical bonding nature of SnO2 is covalent rather than ionic. During sintering, the non-densifying mechanism for mass transport, such as surface diffusion and evaporation-condensation, is dominant, which makes it very difficult to densify pure SnO2. However, this poor densification can be useful for the fabrication of SnO2 with a porous microstructure. Porous SnO2 is often used in electronic devices, such as gas sensors, electrocatalytic electrodes, electro-optical devices, and photovoltaic cells [1-4]. Meanwhile, dense SnO2 ceramics is applicable to other electronic devices like varistors [1].
- Research has shown that the addition of various oxides, such as CoO, CuO, MnO, ZnO, and Nb2O5, to SnO2 leads to high density [4-7]. In the case of CoO-doped SnO2, 99% of the theoretical density was obtained during sintering at 1400°C [7]. In this system, grain boundary diffusion is activated by vacancies near to the grain boundary. Here, vacancies were created by the difference in valence between Sn4+ and Co2+ as a solid solution. In the case of CuO-doped SnO2, shrinkage was initiated at about 1000°C, and high density of 98% of theoretical density was achieved at 1400°C [6]. This system is known as the activated sintering system wherein grain boundary diffusion operates at a low temperature. Further, when doped with TiO2, SnO2 forms an isostructural solid solution with TiO2. Because of this, SnO2 and TiO2 have the same structure as a rutile. In this system, shrinkage also started at about 1000°C during sintering. In this case, operating mass transport mechanism is the grain boundary diffusion as well [2].
- Most electronic devices based on SnO2 have a porous microstructure with a porosity of > 40%, which is very desirable for gas sensor applications [1]. However, for the vast majority of applications involving porous materials, it is desirable to have high mechanical strength paired with high porosity. In the case of pure SnO2, it is necessary to use a high sintering temperature of ~1300°C to obtain a porosity of > 40% [1, 4]. As mentioned previously, the above three systems have the same mass transport path as the grain boundary diffusion at low sintering temperatures. It is well known that the activation energy required in volume diffusion is higher than that required in grain boundary diffusion. This means that temperature sensitivity is higher in grain boundary diffusion than it is in volume diffusion. Therefore, the present study investigated the sintering behavior and dielectric properties of SnO2 ceramic with a porous microstructure while considering temperature sensitivity at a low temperature of 950°C. Sample compositions were chosen as SnO2-CoO(0.3wt%)-CuO(1wt%) with varying contents of TiO2. These compositions were intended to take advantage of the synergistic effect of the grain boundary diffusion. The TiO2 contents were controlled according to the desired microstructure design. As a result, porous microstructures with porosity of ~40% were obtained. The main advantages of this approach are its simplicity, low cost, and its ability to achieve a controlled microstructure by TiO2 content.
1. Introduction
- SnO2 (Samchun pure chemical Co., South Korea; purity: 99.0%), CoO (Sigma Aldrich Co., China: < 325 mesh), CuO (Kojundo chemical Co., Japan; purity: 99.9%, 2 µm), and pure rutile TiO2 (Kojundo chemical Co., Japan; purity: 99.9%, rutile, 2 µm) were obtained as raw materials. Mixed powders of SnO2-CoO(0.3wt%)-CuO(1wt%)-TiO2 were then prepared through wet-milling for 24 h in a polyethylene bottle. Zirconia balls and an ethyl alcohol were used as the milling media and the solvent, respectively. After ball milling, the powders were dried at 90°C for 24 h. The dried powders were then crushed and sieved (500 mesh). Next, ~2 g of each powder was uniaxially pressed into pellets under 0.5 MPa, after which the pellets were cold isostatically pressed under 100 MPa for 3 min. The green pellets had a diameter of ~13 mm and a thickness of 4 mm. The green compact pellets were sintered at 950°C under air in a MoSi2 resistance box furnace. The heating and cooling rates were both ~5°C/min.
- This heating at 950°C proceeded for 8 h. For the samples used in the experiment described herein, the amounts of CoO and CuO were kept at 0.3 and 1 wt%, respectively. The contents of TiO2 were varied up to 0.04 mol for the composition of Sn1-xTixO2. The developed phases of the sintered body were analyzed using standard X-ray diffraction with a diffractometer (D/Max 2000, Rigaku Co., Japan). The sintered densities were obtained by the Archimedes method using distilled water as the immersion medium. Each data point for density was determined based on average measurements of three similar specimens. The BET surface area and BJH pore size distribution were measured with nitrogen adsorption isotherm at 77K using a specific surface area analyzer (3flex).
- The fractured surfaces of the sintered samples were observed through micrographs taken by scanning electron microscopy (VEGA II LMU, TESCAN). The grain sizes were determined from the SEM micrographs using the image analysis software package (VEGA II, TESCAN). To measure capacitance, an Ag electrode material (Ferro Co., USA) was pasted on the polished surface, which was then baked at 400°C for 10 min. The capacitance was measured at room temperature over a frequency range between 102 and 106 Hz using a source meter (2400, Keithley). The dielectric constant was calculated using the following equation εr = Cd/εoA, where εo is the dielectric constant in vacuum, C is the measured value of sample capacitance, and d and A are the thickness and area of the current samples, respectively.
2. Experimental Procedure
- 3.1 The solution effect of TiO2 in SnO2-CoO(0.3wt%)-CuO(1wt%) samples
- Fig. 1 shows the X-ray diffraction patterns of (Sn,Ti)O2-CoO(0.3wt%)-CuO(1wt%) samples with varying TiO2 contents. The specific compositions of the present samples are Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%), where x = 0, 0.01, 0.02, and 0.04. The results only identified SnO2(rutile) and CuO peaks. Therefore, the addition of TiO2 into SnO2 does not lead to the emergence of any second phases, and the TiO2 is dissolved into the SnO2 matrix as (Sn,Ti)O2. The solid solution effect is shown in Fig. 1(b). The SnO2(rutile) peak is slightly shifted to the left (low angle side). This is why the lattice parameters of SnO2 unit cell change in response to the solution of TiO2. Based on the result of Bueno’s work [2], the a and c lattice parameters of the SnO2 unit cell slightly decreased when the TiO2 was solved into the SnO2 matrix. Moreover, CoO is considered to have been dissolved into the SnO2 matrix, because CoO-related secondary phase was not detected under X-ray resolution. The present result is consistent with previously described results [2, 6, 7]. Therefore, the current systems are considered to exist as a mixture of SnO2 and CuO.
- 3.2 Densification
- Pure SnO2 is difficult to sinter, even at the high temperature of 1400°C [2]. However, in the case of the SnO2-CuO system, shrinkage was produced even at a low temperature under 1000°C [6, 8]. The present experiment was carried out at 950°C for 8 h. Fig. 2 shows sintered densities for the SnO2-CoO(0.3wt%)-CuO(1wt%) system with varying TiO2 content. The sintered density was improved with increasing TiO2 content up to 0.04 mol. The X-ray results indicate that the present system consists of the mixture of both SnO2 and CuO.
- The SnO2-CuO system was proven to be an activated sintering system at low temperature [6]. Activated sintering features improved grain boundary diffusion at a low temperature, at which volume (lattice) diffusion is not operable. In this system, CuO serves as an activator to improve the grain boundary diffusion. Therefore, in the case of the current system as well, the improved grain boundary diffusion is considered to be responsible for the enhanced densification at the low temperature, because pure SnO2 has a non-densifying mechanism—involving evaporation-condensation and surface diffusion—even at high temperatures of >1400°C [2]. CoO is also an effective additive to improve the densification of SnO2. In CoO-doped SnO2 matrix, a solid solution is formed by substituting Sn4+ ions for Co2+ or Co3+ ions [1, 7]. According to the defect chemistry, oxygen vacancies near the grain boundary are created by the above substitution, thus leading to enhanced densification of SnO2 through the grain boundary diffusion. From the X-ray data, CoO-related secondary phase was not found, thus indicating that CoO appears to have dissolved into the SnO2 matrix. It is therefore believed that the CoO solution affected the enhanced densification of SnO2 matrix. Further, SnO2 and TiO2 have the same structure as the rutile phase along with the same cation valence of +4. The chemical bonding nature of SnO2 is more covalent than that of TiO2. Therefore, when TiO2 is dissolved into SnO2, isostructural solid solution is formed and oxygen vacancy is not created. However, Sn interstitials and Sn vacancies can be formed near the grain boundaries due to the substituted Sn4+. Based on these relationships, the grain boundary diffusion can be enhanced by the solid solution process at a low temperature, as suggested by Bueno et al [2].
- As described above, the three systems of SnO2-CuO, SnO2-CoO, and SnO2-TiO2 have the same densification mechanism as the grain boundary diffusion at a low temperature. Grain boundary diffusion is well known to have higher temperature sensitivity than lattice diffusion. Therefore, in the context of the three systems considered herein, densification can be synergistically produced at a low temperature. In the present systems, the three additives of CuO, CoO, and TiO2 were seen to have a synergistic effect on the densification for SnO2 at a low temperature of 950°C, using the temperature sensitivity for the grain boundary diffusion. As seen in Fig. 2, the densities improved with increasing TiO2 content.
- 3.3 Microstructure
- For application to a gas sensor based on SnO2, it is desirable to have a porous structure that has porosity of more than 40% [2]. Based on the theoretical density (ρ = 6.94 g/cm3) of the SnO2-CuO sample calculated using the mixture rule, the current samples have ~ 40% porosity, as can be seen in Fig. 2. Fig. 3 shows the BET surface areas of samples with varying TiO2 content. In general, the BET surface area decreased with increasing TiO2 content. This is considered to be the reason why the sample density increased with increasing TiO2 content. Fig. 4 shows a nitrogen adsorption isotherm plot of the samples. The adsorption curves exhibited an abrupt increment at the high pressure range (> 0.9 P/Po) for all samples, indicating the formation of a porous structure in all samples. These results are consistent with those reported for a porous nanocrystalline of SnO2 by Y. Zong et al [9].
- Altogether, introducing porosity into materials resulted in decreased density, which degraded the mechanical strength of the materials. For nearly all applications of porous materials, it is desirable to have both high mechanical strength and high porosity. The mechanical strength for porous materials depends on many parameters, including the grain size, grain bond area, pore size distribution, and porosity [10]. The pore size distribution of the present samples is shown in Fig. 5. All samples appear to have nearly constant pore size distributions. Therefore, grain size and porosity can be considered to have made main contributions to the mechanical strength of the current samples. As can be seen in Fig. 2, porosity decreased with increasing TiO2 content. As shown in Fig. 6, the grain sizes for the samples without and with TiO2 were measured as 172 and 132 nm, respectively. Thus, grain size decreased in the presence of TiO2. Fig. 6 shows the fractured surfaces of samples with and without TiO2. It can be seen that both samples have a porous structure. Therefore, the addition of TiO2 appears to be very useful for controlling the porosity and strength of this porous body.
- 3.4 Dielectric constant
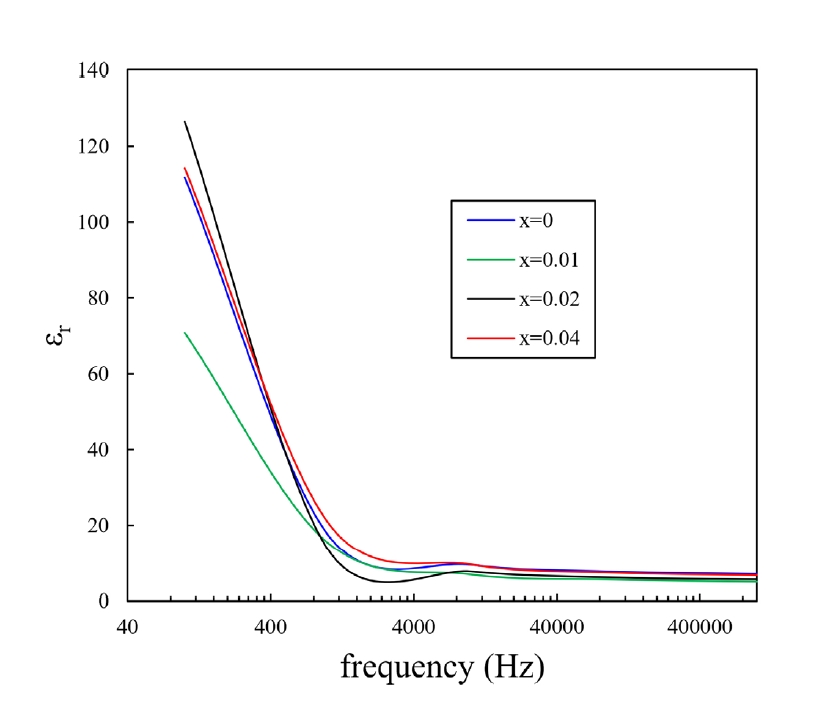
- Dielectric constant is an important parameter for a gas sensing material [1, 11-13]. Fig. 7 shows the relative dielectric constants for the present samples with varying frequencies at room temperature. The static dielectric constants of SnO2 are 24.0 and 23.4 for directions parallel and perpendicular to the tetragonal c-axis, respectively [14]. At the low frequency range below 1kHz, the dielectric constants of all samples were noticeably improved compared to the static dielectric constant. By contrast, the dielectric constant of the samples decreased nearly to the static dielectric constant at high frequencies > 1 kHz.
- Based on the theory of dielectric polarization, there are two polarization processes, including the rotation direction polarization (RDP) process and the space charge polarization (SCP) process [11, 12, 15, 16]. In the case of the RDP process, oxygen vacancies play a role in enhancing the dielectric constant. The oxygen vacancies can exist in the interior and grain boundary of the present samples due to the addition of CoO. The oxygen vacancies can be ionized and then form single and/or double ionized vacancies. Because of this, a large amount of dipole moment can be produced by the combination between positive oxygen vacancies and negative oxygen ions. These dipoles have random directions and cannot be polarized without external electric field. In the presence of external electric field, these dipoles can be poled toward the direction of the external field, inducing the RDP process. The SCP process can also contribute to improving the dielectric constant. There are a lot of defects at grain boundaries in the bulk. Upon the application of external electric field, the space charge in the bulk will move toward the grain boundaries, after which the charges can be trapped by the many defects existing at the grain boundaries, thus inducing a dipole moment. However, at the high frequency range of > 1 kHz, the dielectric response of the RDP and SCP process can break down. Therefore, the dielectric constant of the current samples approached the static dielectric constant. This result is consistent with those obtained in previous investigations [11, 12]. The above result suggests that the densification mechanism for the current samples is the grain boundary diffusion.
3. Results and Discussion
- It is very difficult to densify pure SnO2 with a non-densifying mechanism, even at high temperatures >1400°C. This poor densification property means that SnO2 can be a useful foundation in the fabrication of porous materials. Materials with a porous microstructure with a porosity of >40% are mostly used as electronic devices based on SnO2. In the case of pure SnO2, a high sintering temperature of >1300°C is required to obtain a porosity of > 40%. Therefore, this investigation aimed to both lower the required sintering temperature and enhance the densification, while maintaining a porosity of ~40%. The sample compositions were determined as SnO2-CoO(0.3wt%)-CuO(1wt%) with varying TiO2 contents. The composition appeared to take on the synergistic effect of the grain boundary diffusion during sintering, which is considered to be the reason why the temperature sensitivity of the grain boundary diffusion is higher than that of lattice diffusion. Following the heat-treatment of 950°C for 8h, porous microstructures with a porosity of ~40% were ultimately achieved. This indicates that the synergistic effect of the solid solution and the activator for Sn1-xTixO2 - CoO(0.3wt%)-CuO(1wt%) samples led to better densification at the low temperature of 950°C.
4. Conclusions
-
Acknowledgements
- This research was supported by a Grant from 2023 Research Funds of Andong National University.
Fig. 1.X-ray diffraction patterns of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples: (a) (i) x = 0, (ii) x = 0.01, (iii) x = 0.02, and (iv) x = 0.04; (b) detailed view of the region of 33 – 40°, sintered at 950°C for 8 h.
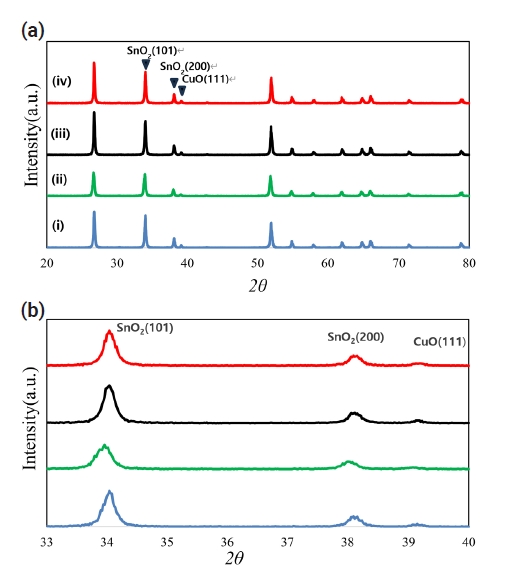

Fig. 2.Sintered densities of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
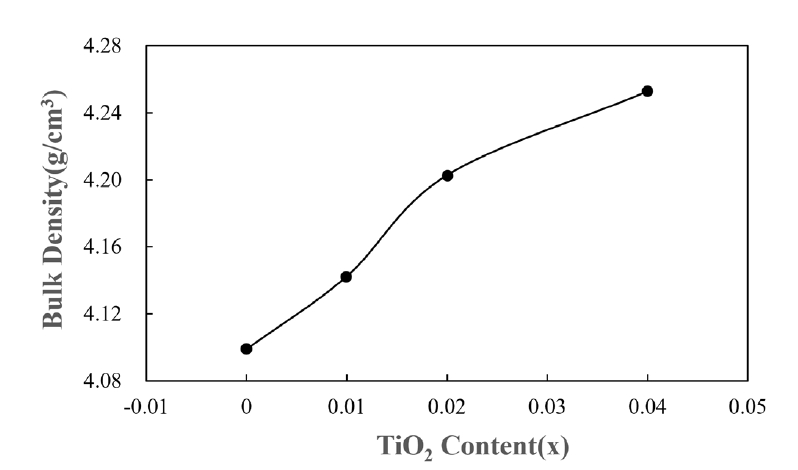

Fig. 3.Brunauer-Emmett-Teller (BET) surface areas of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
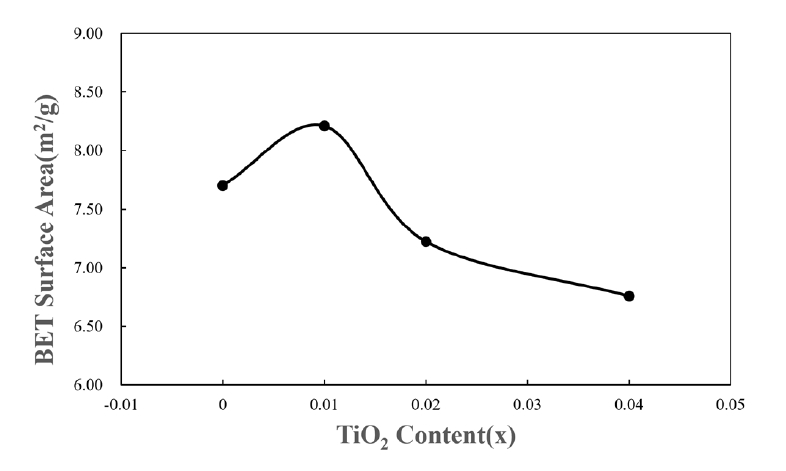

Fig. 4.Nitrogen adsorption isotherm of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.


Fig. 5.Adsorption pore size distributions of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
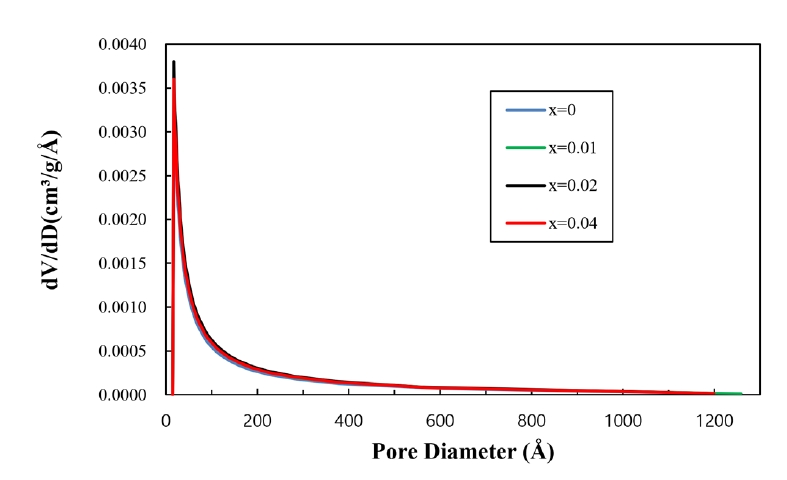

Fig. 6.Scanning electron microscopy (SEM) images of fractured surfaces of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples, sintered at 950°C for 8 h: (a) x = 0 and (b) x = 0.04.
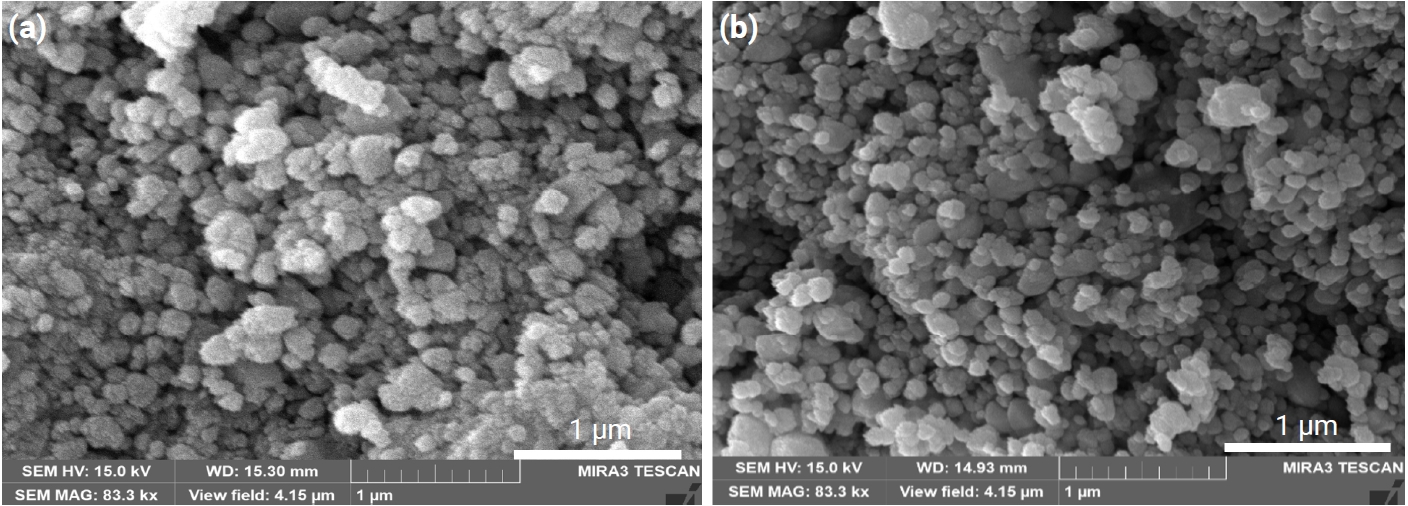

Fig. 7.Dielectric constants with various frequencies of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples at room temperature, sintered at 950°C for 8 h.

- 1. P. R. Bueno and J. A. Varela: Mater. Res., 9 (2006) 293.Article
- 2. P. R. Bueno, E. R. Leite, L. O. S. Bulhoes, E. Longo and C. O. Paiva-Santos: J. Eur. Ceram. Soc., 23 (2003) 887.Article
- 3. M. Radecka, K. Zakrzewska and M. Rekas: Sensors and Actuators., B47 (1998) 194.
- 4. T. Kimura, S. Inada and T. Yamaguchi: J. Mater. Sci., 24 (1989) 220.ArticlePDF
- 5. P. R. Bueno, M. R. Cassia-Santos, L. G. P. Simoes, J. W. Gomes, E. Longo and J. A. Varela: J. Am. Ceram. Soc., 85 (2002) 282.Article
- 6. J. Lalande, R. Ollitrault-Fichet and P. Boch: J. Eur. Ceram. Soc., 20 (2000) 2415.Article
- 7. J. A. Varela, J. A. Cerri, E. R. Leite, E. Longo, M. Shamsuzzoha and R. C. Bradt: Ceram. Int., 25 (1999) 253.Article
- 8. N. Dolet, J. M. Heintz, L. Rabardel, M. Onillon and J. P. Bonnet: J. Mater. Sci., 30 (1995) 365.ArticlePDF
- 9. Y. Zong, Y. Cao, D. Jia and P. Hu: Sensors and Actuators., B 145 (2010) 84.
- 10. Y. K. Paek, K. S. Oh and H. J. Lee: J. Kor. Ceram. Soc., 44 (2007) 79.Article
- 11. P. G. Li, X. Guo, X. F. Wang and W. H. Tang: J. Alloys Compd., 479 (2009) 74.Article
- 12. M. S. Castro and C. M. Aldao: J. Euro. Ceram. Soc., 18 (1998) 2233.Article
- 13. K. Lee, M. Sahu, S. Hajra, K. Mohanta and H. J. Kim: Ceram. Int., 47 (2021) 22794.Article
- 14. H. J. van Daal: J. Appl. Phys., 39 (1968) 4467.
- 15. S. M. Zhou, Y. S. Feng and L. D. Zhang: Chem. Phys. Lett., 369 (2003) 610.Article
- 16. B. Song, J. K. Jian, G. Wang, M. Lei, H. Q. Bao and X. L. Chen: J. Alloys Compd., 460 (2008) 31.Article
References
Figure & Data
References
Citations
Citations to this article as recorded by 

The Effect of TiO2 Addition on Low-temperature Sintering Behaviors in a SnO2-CoO-CuO System

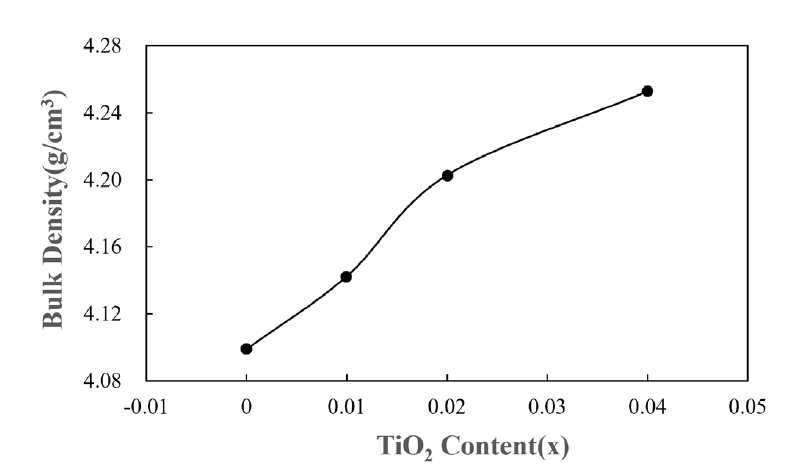
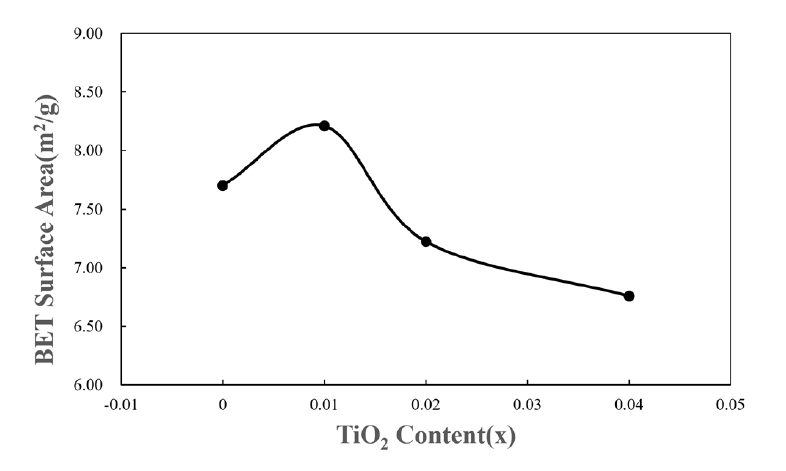
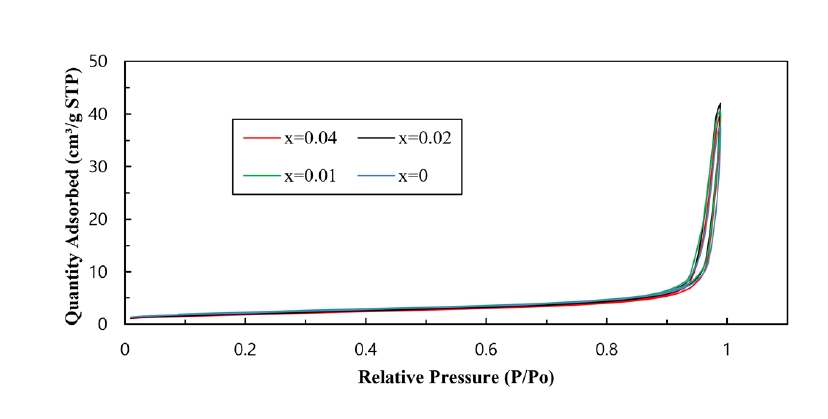

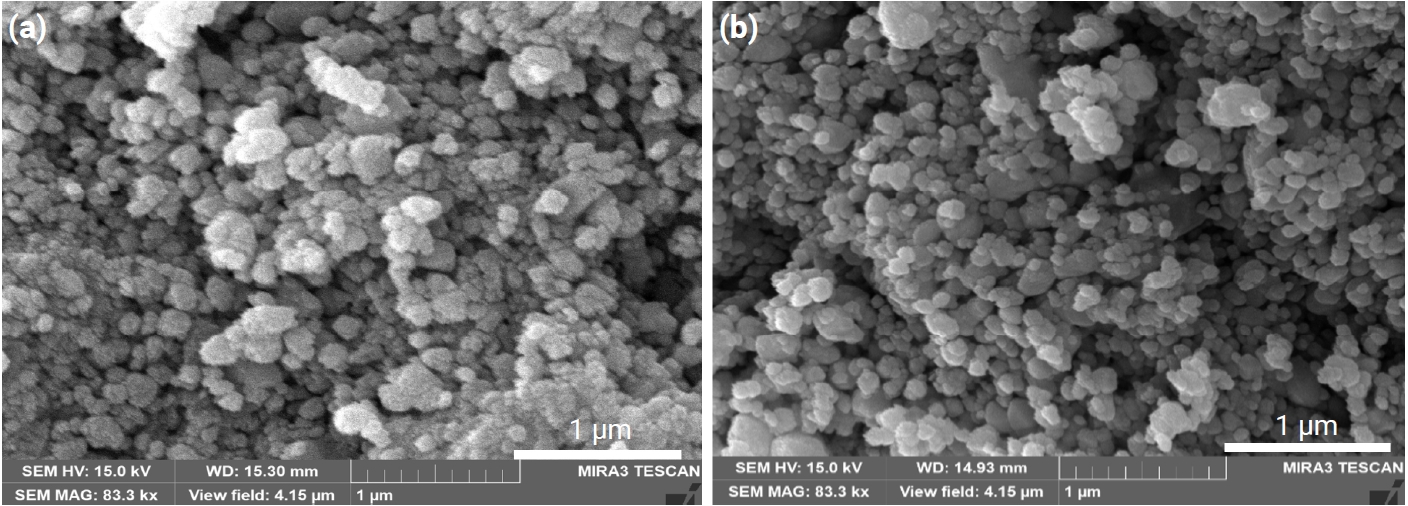
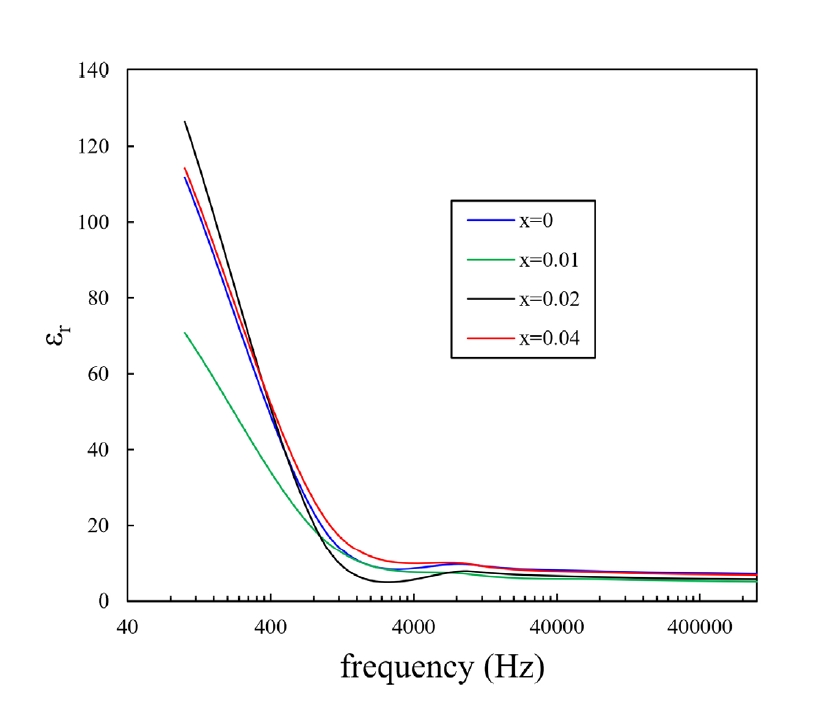
Fig. 1. X-ray diffraction patterns of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples: (a) (i) x = 0, (ii) x = 0.01, (iii) x = 0.02, and (iv) x = 0.04; (b) detailed view of the region of 33 – 40°, sintered at 950°C for 8 h.
Fig. 2. Sintered densities of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
Fig. 3. Brunauer-Emmett-Teller (BET) surface areas of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
Fig. 4. Nitrogen adsorption isotherm of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
Fig. 5. Adsorption pore size distributions of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples with TiO2 content, sintered at 950°C for 8 h.
Fig. 6. Scanning electron microscopy (SEM) images of fractured surfaces of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples, sintered at 950°C for 8 h: (a) x = 0 and (b) x = 0.04.
Fig. 7. Dielectric constants with various frequencies of Sn1-xTixO2-CoO(0.3wt%)-CuO(1wt%) samples at room temperature, sintered at 950°C for 8 h.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
The Effect of TiO2 Addition on Low-temperature Sintering Behaviors in a SnO2-CoO-CuO System
TOP
 KPMI
KPMI

 ePub Link
ePub Link Cite this Article
Cite this Article







