- [English]
- A Separator with Activated Carbon Powder Layer to Enhance the Performance of Lithium-Sulfur Batteries
-
Duc-Luong Vu, Jae-Won Lee
-
J Korean Powder Metall Inst. 2018;25(6):466-474. Published online December 1, 2018
-
DOI: https://doi.org/10.4150/KPMI.2018.25.6.466
-
-
1,130
View
-
4
Download
-
2
Citations
-
 Abstract Abstract
 PDF PDF
The high theoretical energy density (2600 Wh kg−1) of Lithium-sulfur batteries and the high theoretical capacity of elemental sulfur (1672 mAh g−1) attract significant research attention. However, the poor electrical conductivity of sulfur and the polysulfide shuttle effect are chronic problems resulting in low sulfur utilization and poor cycling stability. In this study, we address these problems by coating a polyethylene separator with a layer of activated carbon powder. A lithium-sulfur cell containing the activated carbon powder-coated separator exhibits an initial specific discharge capacity of 1400 mAh g−1 at 0.1 C, and retains 63% of the initial capacity after 100 cycles at 0.2 C, whereas the equivalent cell with a bare separator exhibits a 1200 mAh g−1 initial specific discharge capacity, and 50% capacity retention under the same conditions. The activated carbon powder-coated separator also enhances the rate capability. These results indicate that the microstructure of the activated carbon powder layer provides space for the sulfur redox reaction and facilitates fast electron transport. Concurrently, the activated carbon powder layer traps and reutilizes any polysulfides dissolved in the electrolyte. The approach presented here provides insights for overcoming the problems associated with lithium-sulfur batteries and promoting their practical use. -
Citations
Citations to this article as recorded by  - A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
Yueting Zhu, Jingjing Wang, Yanshu Wang, Ying Zhu, Yixuan Li, Shicheng Zhao
Ionics.2022; 28(4): 1693. CrossRef - High thermal stability multilayered electrolyte complexes via layer-by-layer for long-life lithium-sulfur battery
Jing Wang, Yufan Li, Xianmei Deng, Lei Yan, Zhiqiang Shi
Ionics.2020; 26(11): 5481. CrossRef
- [English]
- Lithium-silicate coating on Lithium Nickel Manganese Oxide (LiNi0.7Mn0.3O2) with a Layered Structure
-
Dong-jin Kim, Da-ye Yoon, Woo-byoung Kim, Jae-won Lee
-
J Korean Powder Metall Inst. 2017;24(2):87-95. Published online April 1, 2017
-
DOI: https://doi.org/10.4150/KPMI.2017.24.2.87
-
-
1,318
View
-
7
Download
-
1
Citations
-
 Abstract Abstract
 PDF PDF
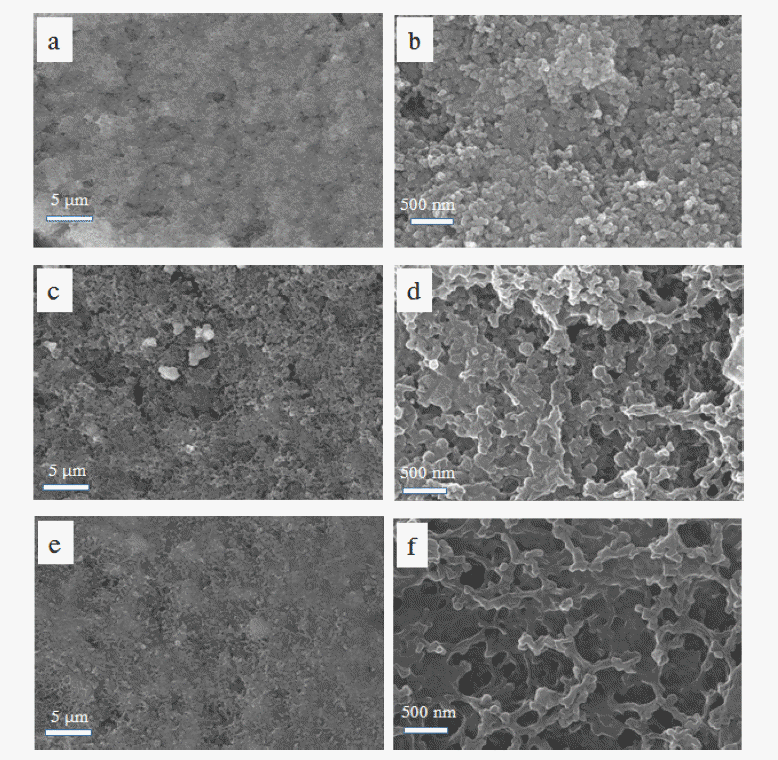
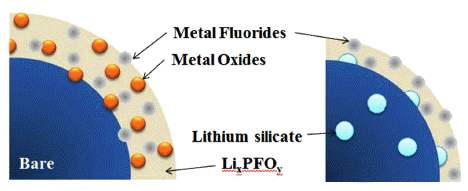
Lithium silicate, a lithium-ion conducting ceramic, is coated on a layer-structured lithium nickel manganese oxide (LiNi0.7Mn0.3O2). Residual lithium compounds (Li2CO3 and LiOH) on the surface of the cathode material and SiO2 derived from tetraethylorthosilicate are used as lithium and silicon sources, respectively. Powder X-ray diffraction and scanning electron microscopy with energy-dispersive spectroscopy analyses show that lithium silicate is coated uniformly on the cathode particles. Charge and discharge tests of the samples show that the coating can enhance the rate capability and cycle life performance. The improvements are attributed to the reduced interfacial resistance originating from suppression of solid-electrolyte interface (SEI) formation and dissolution of Ni and Mn due to the coating. An X-ray photoelectron spectroscopy study of the cycled electrodes shows that nickel oxide and manganese oxide particles are formed on the surface of the electrode and that greater decomposition of the electrolyte occurs for the bare sample, which confirms the assumption that SEI formation and Ni and Mn dissolution can be reduced using the coating process. -
Citations
Citations to this article as recorded by  - Artificial cathode-electrolyte interphases on nickel-rich cathode materials modified by silyl functional group
Hye Ji Song, Seol Heui Jang, Juhyeon Ahn, Si Hyoung Oh, Taeeun Yim
Journal of Power Sources.2019; 416: 1. CrossRef
- [English]
- Preparation and Characterization of Porous Silicon and Carbon Composite as an Anode Material for Lithium Rechargeable Batteries
-
Junsoo Park, Jae-won Lee
-
J Korean Powder Metall Inst. 2015;22(1):15-20. Published online February 1, 2015
-
DOI: https://doi.org/10.4150/KPMI.2015.22.1.15
-
-
 Abstract Abstract
 PDF PDF
The composite of porous silicon (Si) and amorphous carbon (C) is prepared by pyrolysis of a nano-porous Si + pitch mixture. The nano-porous Si is prepared by mechanical milling of magnesium powder with silicon monoxide (SiO) followed by removal of MgO with hydrochloric acid (etching process). The Brunauer-Emmett-Teller (BET) surface area of porous Si (64.52 m2g−1) is much higher than that before etching Si/MgO (4.28 m2g−1) which indicates pores are formed in Si after the etching process. Cycling stability is examined for the nano-porous Si + C composite and the result is compared with the composite of nonporous Si + C. The capacity retention of the former composite is 59.6% after 50 charge/discharge cycles while the latter shows only 28.0%. The pores of Si formed after the etching process is believed to accommodate large volumetric change of Si during charging and discharging process.
- [Korean]
- Research Trend of Electrode Materials for Lithium Rechargeable Batteries
-
Jae-won Lee, Woo-Byoung Kim
-
J Korean Powder Metall Inst. 2014;21(6):473-479. Published online December 1, 2014
-
DOI: https://doi.org/10.4150/KPMI.2014.21.6.473
-
-
3,714
View
-
65
Download
-
7
Citations
-
 PDF PDF
-
Citations
Citations to this article as recorded by  - A neuro-fuzzy system to evaluate the remaining useful life of the lithium-ion battery using the impedance spectrum in the overall range of SOCs
Min-Seong Kim, Abdul Shakoor Akram, Woojin Choi
Journal of Power Electronics.2025; 25(1): 103. CrossRef - Highly Stable and Rechargeable Lithium‐Ion Battery Using a Designed Organic PTVE‐Impregnated Porous Graphitic Carbon Composite
Ji-Won Son, Jae Seob Lee, Hyun Ho Choi, Fanglin Wu, Hong-Il Kim, Tae Ju Kang, Hyun Woo Kim, Shan Fang, Ying Liu, Jung Sang Cho, Amar Patil
International Journal of Energy Research.2025;[Epub] CrossRef - Hydrogen Reduction Behavior of NCM-based Lithium-ion Battery Cathode Materials
So-Yeong Lee, So-Yeon Lee, Dae-Hyeon Lee, Ho-Sang Sohn
journal of Korean Powder Metallurgy Institute.2024; 31(2): 163. CrossRef - Effect of Calcination Temperature on Ionic Conductivity of All-solid State Battery Electrolytes
Yu Taek Hong, Ji Min Im, Ki Sang Baek, Chan Gyu Kim, Seung Wook Baek, Jung Hyun Kim
New & Renewable Energy.2024; 20(2): 71. CrossRef - FeCO3/rGO Composites Prepared by CO2 Capture and Carbonate Conversion: Anode Material in Lithium-Ion Batteries with Enhanced Performance
Sanglim Lee, Gahwi Gu, Daeung Park, Hwiyun Im, Yongjae Lee, Junseok Moon, Weonho Shin, Hiesang Sohn
Membrane Journal.2024; 34(5): 253. CrossRef - Research Trend in Rock Salt Structured High Entropy Cathode
Minjeong Kim, Jahun Koo, Minjeong Kang, Juah Song, Chunjoong Kim
Ceramist.2022; 25(1): 90. CrossRef - Recovery of Co and Ni from Strong Acidic Solution by Cyanex 301
Yeon-Chul Cho, Ki-Hun Kim, Jae-Woo Ahn
Resources Recycling.2021; 30(6): 28. CrossRef
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
-
Jae-won Lee, Dae Weon Kim, Seong Tae Jang
-
J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
-
DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
-
-
 Abstract Abstract
 PDF PDF
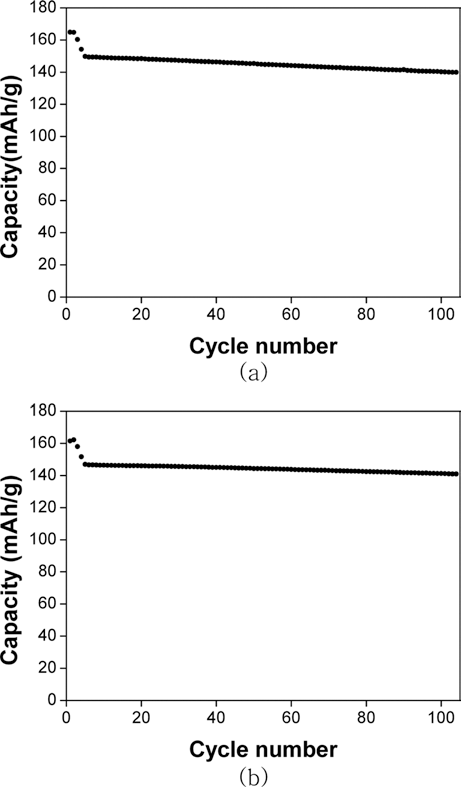
Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
|