Search
- Page Path
- HOME > Search
- [Korean]
- Study on Manufacture of High Purity TiCl4 and Synthesis of High Purity Ti Powders
- Jieun Lee, Jin-Ho Yoon, Chan Gi Lee
- J Korean Powder Metall Inst. 2019;26(4):282-289. Published online August 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.4.282
- 1,359 View
- 23 Download
-
 Abstract
Abstract
 PDF
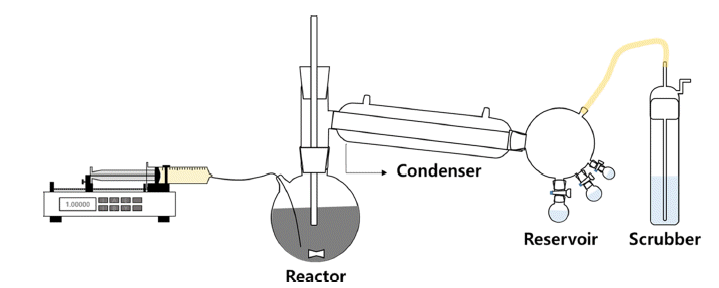
PDF Ti has received considerable attention for aerospace, vehicle, and semiconductor industry applications because of its acid-resistant nature, low density, and high mechanical strength. A common precursor used for preparing Ti materials is TiCl4. To prepare high-purity TiCl4, a process based on the removal of VOCl3 has been widely applied. However, VOCl3 removal by distillation and condensation is difficult because of the similar physical properties of TiCl4 and VOCl3. To circumvent this problem, in this study, we have developed a process for VOCl3 removal using Cu powder and mineral oil as purifying agents. The effects of reaction time and temperature, and ratio of purifying agents on the VOCl3 removal efficiency are investigated by chemical and structural measurements. Clear TiCl4 is obtained after the removal of VOCl3. Notably, complete removal of VOCl3 is achieved with 2.0 wt% of mineral oil. Moreover, the refined TiCl4 is used as a precursor for the synthesis of Ti powder. Ti powder is fabricated by a thermal reduction process at 1,100ºC using an H2-Ar gas mixture. The average size of the Ti powder particles is in the range of 1-3 μm.
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 853 View
- 4 Download
-
 Abstract
Abstract
 PDF
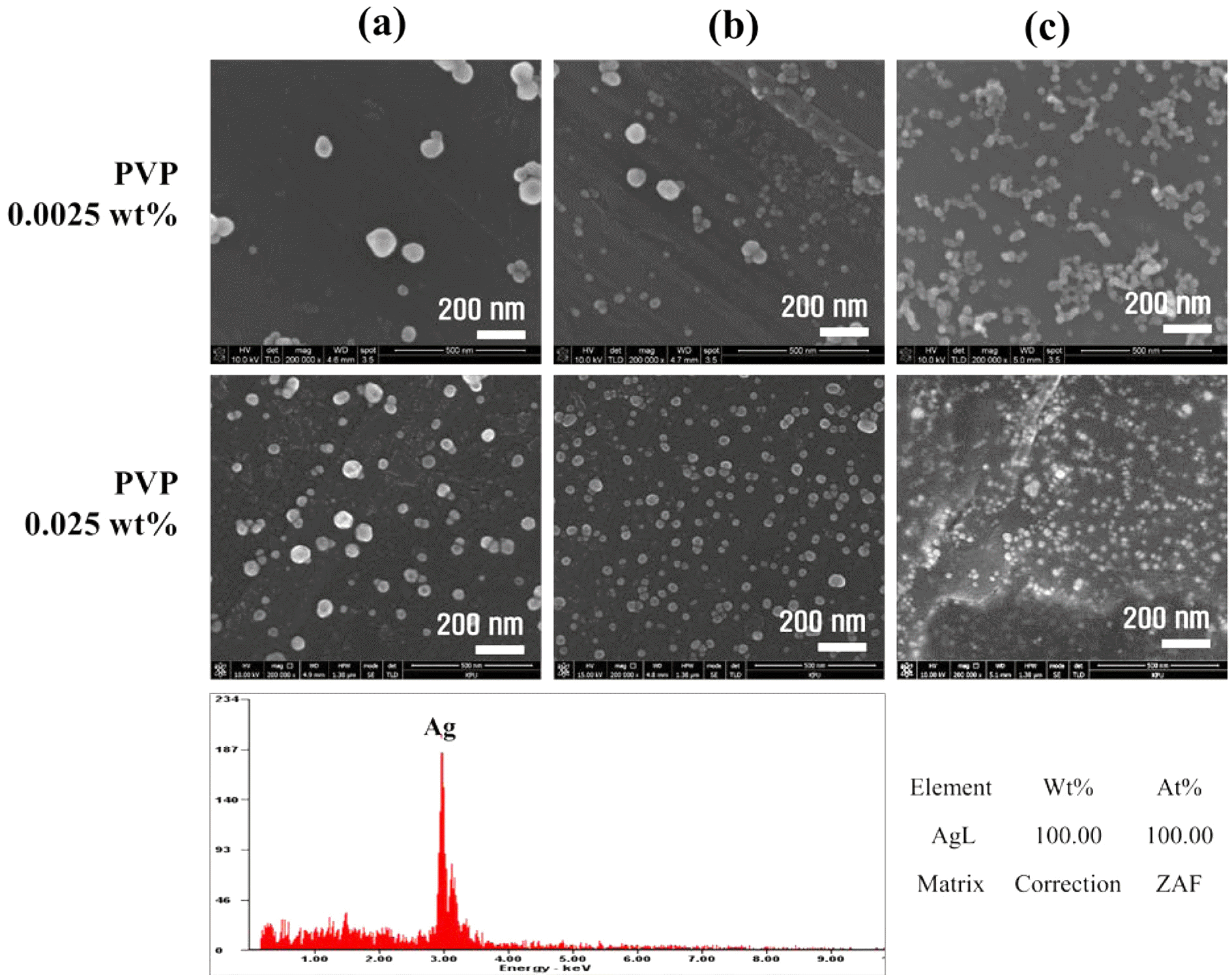
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- Synthesis and analysis CdSe/ZnS quantum dot with a Core/shell Continuous Synthesis System Using a Microfluidic Reactor
- Myung Hwan Hong, So Young Joo, Lee-Seung Kang, Chan Gi Lee
- J Korean Powder Metall Inst. 2018;25(2):132-136. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.132
- 1,892 View
- 10 Download
-
 Abstract
Abstract
 PDF
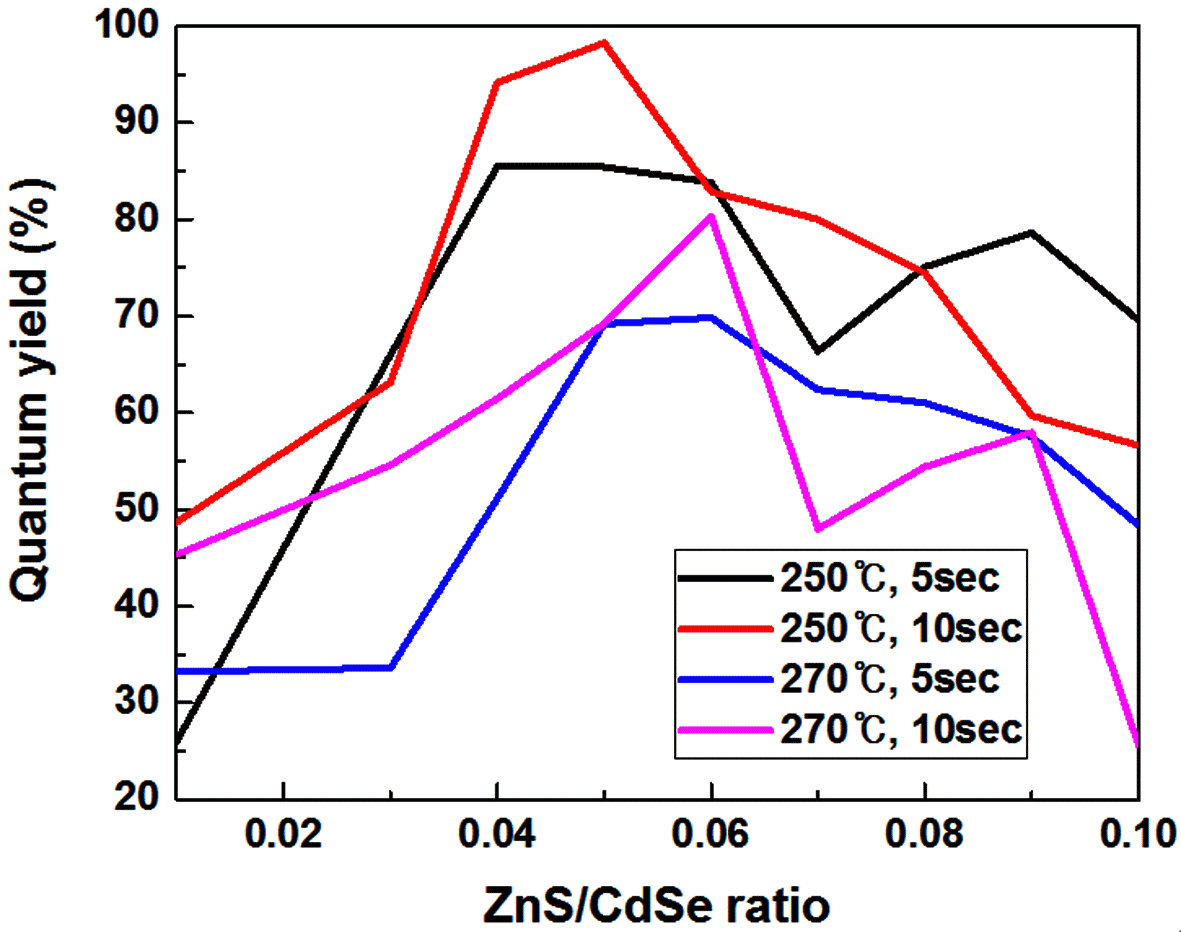
PDF Core/shell CdSe/ZnS quantum dots (QDs) are synthesized by a microfluidic reactor-assisted continuous reactor system. Photoluminescence and absorbance of synthesized CdSe/ZnS core/shell QDs are investigated by fluorescence spectrophotometry and online UV-Vis spectrometry. Three reaction conditions, namely; the shell coating reaction temperature, the shell coating reaction time, and the ZnS/CdSe precursor volume ratio, are combined in the synthesis process. The quantum yield of the synthesized CdSe QDs is determined for each condition. CdSe/ZnS QDs with a higher quantum yield are obtained compared to the discontinuous microfluidic reactor synthesis system. The maximum quantum efficiency is 98.3% when the reaction temperature, reaction time, and ZnS/CdSe ratio are 270°C, 10 s, and 0.05, respectively. Obtained results indicate that a continuous synthesis of the Core/shell CdSe/ZnS QDs with a high quantum efficiency could be achieved by isolating the reaction from the external environment.
- [Korean]
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,001 View
- 8 Download
-
 Abstract
Abstract
 PDF
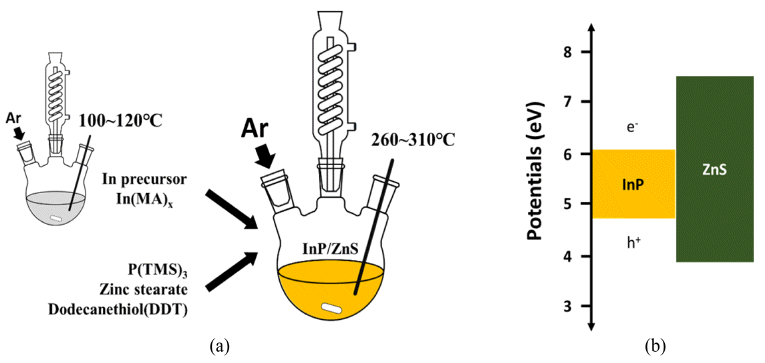
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
- Synthesis and analysis CdSe Quantum dot with a Microfluidic Reactor Using a Combinatorial Synthesis System
- Myung Hwan Hong, Duk-Hee Lee, Lee-Seung Kang, Chan Gi Lee, Bum-Sung Kim, Nam-Hoon Kim
- J Korean Powder Metall Inst. 2016;23(2):143-148. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.143
- 851 View
- 7 Download
-
 Abstract
Abstract
 PDF
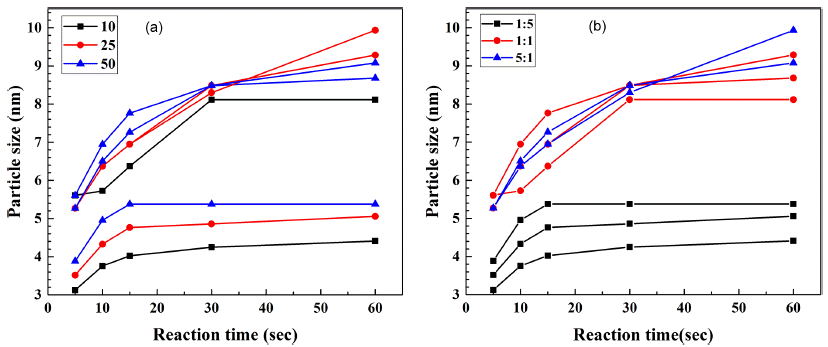
PDF A microfluidic reactor with computer-controlled programmable isocratic pumps and online detectors is employed as a combinatorial synthesis system to synthesize and analyze materials for fabricating CdSe quantum dots for various applications. Four reaction condition parameters, namely, the reaction temperature, reaction time, Cd/Se compositional ratio, and precursor concentration, are combined in synthesis condition sets, and the size of the synthesized CdSe quantum dots is determined for each condition. The average time corresponding to each reaction condition for obtaining the ultraviolet–visible absorbance and photoluminescence spectra is approximately 10 min. Using the data from the combinatorial synthesis system, the effects of the reaction conditions on the synthesized CdSe quantum dots are determined. Further, the data is used to determine the relationships between the reaction conditions and the CdSe particle size. This method should aid in determining and selecting the optimal conditions for synthesizing nanoparticles for diverse applications.
- [Korean]
- Leaching behavior of Ga and In from MOCVD dust
- Kyung-Soo Park, Basudev Swain, Lee Seung Kang, Chan Gi Lee, Hyun Seon Hong, Jong-Gil Shim, Jeung-Jin Park
- J Korean Powder Metall Inst. 2014;21(3):202-206. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.202
- 971 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
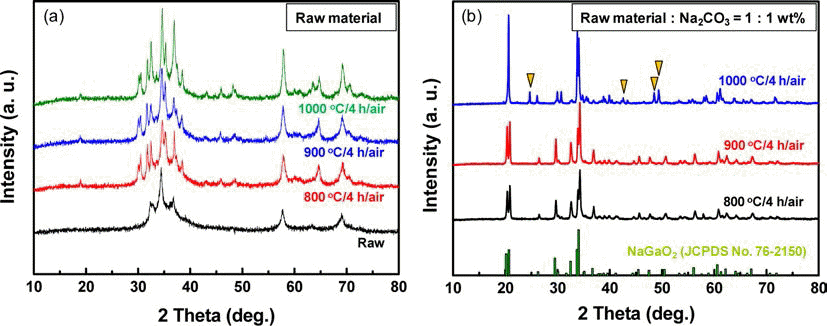
PDF Leaching of MOCVD dust in the LED industry is an essential stage for hydro-metallurgical recovery of pure Ga and In. To recover Ga and In, the leaching behavior of MOCVD scrap of an LED, which contains significant amounts of Ga, In, Al and Fe in various phases, has been investigated. The leaching process must be performed effectively to maximize recovery of Ga and In metals using the most efficient lixiviant. Crystalline structure and metallic composition of the raw MOCVD dust were analyzed prior to digestion. Subsequently, various mineral acids were tested to comprehensively study and optimize the leaching parameters such as acidity, pulp density, temperature and time. The most effective leaching of Ga and In was observed for a boiling 4 M HCl solution vigorously stirred at 400 rpm. Phase transformation of GaN into gallium oxide by heat treatment also improved the leaching efficiency of Ga. Subsequently high purity Ga and In can be recovered by series of hydro processes.
-
Citations
Citations to this article as recorded by- Selective Solvent Extraction of In from Synthesis Solution of MOCVD Dust using D2EHPA
Byoungyong Im, Basudev Swain, Chan Gi Lee, Jae Layng Park, Kyung-Soo Park, Jong-Gil Shim, Jeung-Jin Park
Journal of the Korean Institute of Resources Recycling.2015; 24(5): 80. CrossRef - Fabrication of High Purity Ga-containing Solution using MOCVD dust
Duk-Hee Lee, Jin-Ho Yoon, Kyung-Soo Park, Myung-Hwan Hong, Chan-Gi Lee, Jeung-Jin Park
Journal of the Korean Institute of Resources Recycling.2015; 24(4): 50. CrossRef - Influence of Oxidation Temperatures on the Structure and the Microstructure of GaN MOCVD Scraps
Hyun Seon Hong, Joong Woo Ahn
journal of Korean Powder Metallurgy Institute.1970; 22(4): 278. CrossRef
- Selective Solvent Extraction of In from Synthesis Solution of MOCVD Dust using D2EHPA
TOP
 KPMI
KPMI


 First
First Prev
Prev


