Search
- Page Path
- HOME > Search
- [Korean]
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(4):324-328. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00199

- 1,176 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - This study analyzed the influence of ball size and process control agents on the refinement and dehydrogenation behavior of TiH2 powder. Powders milled using ZrO2 balls with diameters of 0.1 mm, 0.3 mm, and 0.3+0.5+1 mm exhibited a bimodal particle size distribution, of which the first mode had the smallest size of 0.23 μm for the 0.3 mm balls. Using ethanol and/or stearic acid as process control agents was effective in particle refinement. Thermogravimetric analysis showed that dehydrogenation of the milled powder started at a relatively low temperature compared to the raw powder, which is interpreted to have resulted from a decrease in particle size and an increase in defects. The dehydrogenation kinetics of the TiH2 powder were evaluated by the magnitude of peak shift with heating rates using thermogravimetric analysis. The activation energy of the dehydrogenation reaction, calculated from the slope of the Kissinger plot, was measured to be 228.6 kJ/mol for the raw powder and 194.5 kJ/mol for the milled powder. TEM analysis revealed that both the milled and dehydrogenated powders showed an angular shape with a size of about 200 nm.
- [Korean]
- Effect of Heat Treatment Temperature and Atmosphere on the Microstructure of TiH2-WO3 Powder Mixtures
- Han-Eol Lee, Yeon Su Kim, Sung-Tag Oh
- J Korean Powder Metall Inst. 2017;24(1):41-45. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.41

- 849 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
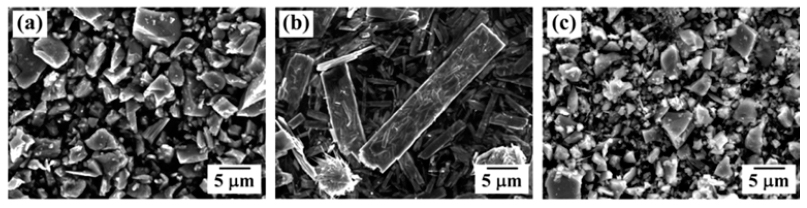
PDF The effects of the heat treatment temperature and of the atmosphere on the dehydrogenation and hydrogen reduction of ball-milled TiH2-WO3 powder mixtures are investigated for the synthesis of Ti-W powders with controlled microstructure. Homogeneously mixed powders with refined TiH2 particles are successfully prepared by ball milling for 24 h. X-ray diffraction (XRD) analyses show that the powder mixture heat-treated in Ar atmosphere is composed of Ti, Ti2O, and W phases, regardless of the heat treatment temperature. However, XRD results for the powder mixture, heat-treated at 600°C in a hydrogen atmosphere, show TiH2 and TiH peaks as well as reaction phase peaks of Ti oxides and W, while the powder mixture heat-treated at 900°C exhibits only XRD peaks attributed to Ti oxides and W. The formation behavior of the reaction phases that are dependent on the heat treatment temperature and on the atmosphere is explained by thermodynamic considerations for the dehydrogenation reaction of TiH2, the hydrogen reduction of WO3 and the partial oxidation of dehydrogenated Ti.
-
Citations
Citations to this article as recorded by- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
Hyunji Kang, Sung Hyun Park, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 472. CrossRef
- Fabrication of Porous W-Ti by Freeze-Drying and Hydrogen Reduction of WO3-TiH2 Powder Mixtures
- [Korean]
- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
- Ki Cheol Jeon, Sung Hyun Park, Na-Yeon Kwon, Sung-Tag Oh
- J Korean Powder Metall Inst. 2016;23(4):303-306. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.303
- 919 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
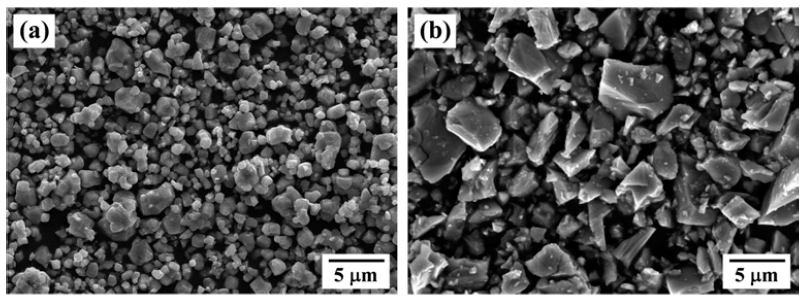
PDF An optimum route to synthesize Ti-Mo system powders is investigated by analyzing the effect of the heat treatment atmosphere on the formation of the reaction phase by dehydrogenation and hydrogen reduction of ball-milled TiH2-MoO3 powder mixtures. Homogeneous powder mixtures with refined particles are prepared by ball milling for 24 h. XRD analysis of the heat-treated powder in a hydrogen atmosphere shows TiH2 and MoO3 peaks in the initial powders as well as the peaks corresponding to the reaction phase species, such as TiH0.7, TiO, MoO2, Mo. In contrast, powder mixtures heated in an argon atmosphere are composed of Ti, TiO, Mo and MoO3 phases. The formation of reaction phases dependent on the atmosphere is explained by the partial pressure of H2 and the reaction temperature, based on thermodynamic considerations for the dehydrogenation reaction of TiH2 and the reduction behavior of MoO3.
-
Citations
Citations to this article as recorded by- Effect of titanium addition on mechanical properties of Mo-Si-B alloys
Won June Choi, Seung Yeong Lee, Chun Woong Park, Jung Hyo Park, Jong Min Byun, Young Do Kim
International Journal of Refractory Metals and Hard Materials.2019; 80: 238. CrossRef
- Effect of titanium addition on mechanical properties of Mo-Si-B alloys
- [Korean]
- Synthesis of NiTi Alloy Powder by the Reaction of NiO-TiH2 Mixing Powders
- Ki Cheol Jeon, Han-Eol Lee, Da-Mi Yim, Sung-Tag Oh
- J Korean Powder Metall Inst. 2015;22(4):266-270. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.266
- 825 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The synthesis of NiTi alloy powders by hydrogen reduction and dehydrogenation process of NiO and TiH2 powder mixtures is investigated. Mixtures of NiO and TiH2 powders are prepared by simple mixing for 1 h or ball milling for 24 h. Simple-mixed mixture shows that fine NiO particles are homogeneously coated on the surface of TiH2 powders, whereas ball milled one exhibits the morphology with mixing of fine NiO and TiH2 particles. Thermogravimetric analysis in hydrogen atmosphere reveals that the NiO and TiH2 phase are changed to metallic Ni and Ti in the temperature range of 260 to 290°C and 553 to 639°C, respectively. In the simple-mixed powders by heat-up to 700°C, agglomerates with solid particles and solidified liquid phase are observed, and the size of agglomerates is increased at 1000°C. From the XRD analysis, the presence of liquid phase is explained by the formation and melting of NiTi2 intermetallic compound due to an exothermic reaction between Ni and Ti. The simple-mixed powders, heated to 1000°C, lead to the formation of NiTi phase but additional Ni-, Ti-rich and Ti-oxide phases. In contrast, the microstructure of ball-milled powders is characterized by the neck-grown particles, forming Ni3Ti, Ti-oxide and unreacted Ni phase.
-
Citations
Citations to this article as recorded by- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
Ki Cheol Jeon, Sung Hyun Park, Na-Yeon Kwon, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 303. CrossRef
- Effect of Heat Treatment Atmosphere on the Microstructure of TiH2-MoO3 Powder Mixtures
TOP
 KPMI
KPMI


 First
First Prev
Prev


