Search
- Page Path
- HOME > Search
- [Korean]
- Inorganic Compound and Cycloserine Composite Particles for Improved Stability
- Dongwon Kim, Heeseo Kim, Hongjun Yoon, Hyuk Jun Cho, Sung Giu Jin
- J Powder Mater. 2024;31(2):126-131. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00002
- 1,808 View
- 31 Download
-
 Abstract
Abstract
 PDF
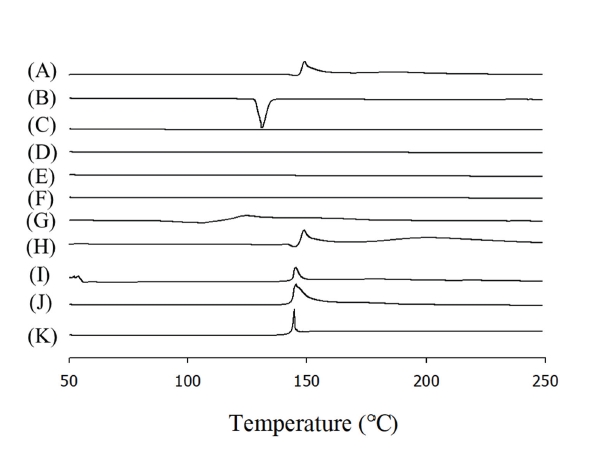
PDF - The aim of this study was to improve the chemical stability of cycloserine containing organic and inorganic compounds. Composite particles were manufactured with a 1:1 weight ratio of organic/inorganic compounds and cycloserine. The influence of organic/inorganic compounds on the stability of cycloserine was investigated under accelerated stress conditions at 60°C/75% RH for 24 hours. In addition, the properties of the composite particles were evaluated using differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and the dissolution of the drug was assessed by preparing it as a hard capsule. Among the organic and inorganic compounds investigated, calcium hydroxide most improved the stability of cycloserine under accelerated stress conditions (53.3 ± 2.2% vs 1.7 ± 0.2%). DSC results confirmed the compatibility between calcium hydroxide and the cycloserine, and SEM results confirmed that it was evenly distributed around the cycloserine. Calcium hydroxide also showed more than 90% cycloserine dissolution within 15 minutes. Therefore, the calcium hydroxide and cycloserine composite particles may be candidates for cycloserine oral pharmaceuticals with enhanced drug stability.
- [Korean]
- Fabrication and Evaluation of Levosulpiride-loaded Amorphous Spray-dried Microparticle for Improved Solubility
- Sung Giu Jin
- J Powder Mater. 2023;30(1):47-52. Published online February 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.1.47
- 838 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF The purpose of this study is to develop and evaluate amorphous spray-dried microparticles (SDM) containing levosulpiride to increase its solubility. SDM are prepared via solvent evaporation using polyvinylpyrrolidone (PVP) as the water-soluble polymer and Cremophor RH40 as the surfactant. The SDM is prepared by varying the amounts of PVP and Cremophor RH40, and its physicochemical properties, solubility, and dissolution are confirmed. All levosulpiride-loaded SDMs converted the crystalline drug into an amorphous form, significantly improving drug solubility and dissolution compared with the drug alone. SDM consisting of drug/PVP/Cremophor RH40 in a weight ratio of 5:10:3, with increased solubility (720 ± 36 vs. 1822 ± 51 μg/mL) and dissolution rate (10.3 ± 2.2 vs. 92.6 ± 6.0%) compared with drug alone, shows potential as a commercial drug for improved oral bioavailability of levosulpiride.
- [Korean]
- Analysis of Wafer Cleaning Solution Characteristics and Metal Dissolution Behavior according to the Addition of Chelating Agent
- Myungsuk Kim, Keunhyuk Ryu, Kun-Jae Lee
- J Korean Powder Metall Inst. 2021;28(1):25-30. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.25

- 1,004 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF The surface of silicon dummy wafers is contaminated with metallic impurities owing to the reaction with and adhesion of chemicals during the oxidation process. These metallic impurities negatively affect the device performance, reliability, and yield. To solve this problem, a wafer-cleaning process that removes metallic impurities is essential. RCA (Radio Corporation of America) cleaning is commonly used, but there are problems such as increased surface roughness and formation of metal hydroxides. Herein, we attempt to use a chelating agent (EDTA) to reduce the surface roughness, improve the stability of cleaning solutions, and prevent the re-adsorption of impurities. The bonding between the cleaning solution and metal powder is analyzed by referring to the Pourbaix diagram. The changes in the ionic conductivity, H2O2 decomposition behavior, and degree of dissolution are checked with a conductivity meter, and the changes in the absorbance and particle size before and after the reaction are confirmed by ultraviolet-visible spectroscopy (UV-vis) and dynamic light scattering (DLS) analyses. Thus, the addition of a chelating agent prevents the decomposition of H2O2 and improves the life of the silicon wafer cleaning solution, allowing it to react smoothly with metallic impurities.
- [English]
- Production of Porous Metallic Glass Granule by Optimizing Chemical Processing
- Song-Yi Kim, Bo-Kyung Guem, Min-Ha Lee, Taek-Soo Kim, Jurgen Eckert, Bum-Sung Kim
- J Korean Powder Metall Inst. 2014;21(4):251-255. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.251
- 1,107 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
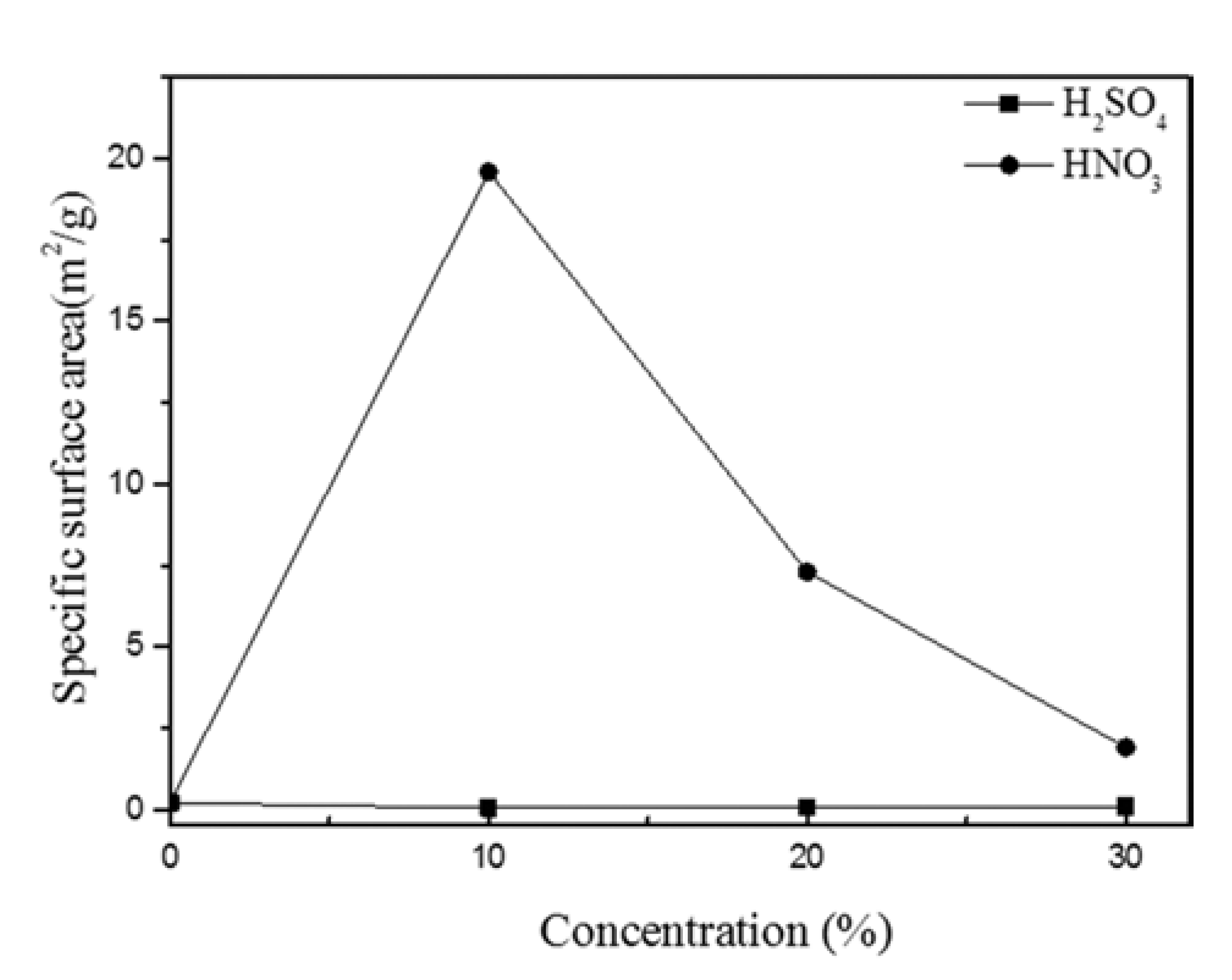
PDF In this study, we optimized dissolution the dissolution conditions of porous amorphous powder to have high specific surface area. Porous metallic glass(MG) granules were fabricated by selective phase dissolution, in which brass is removed from a composite powder consisting of MG and 40 vol.% brass. Dissolution was achieved through various concentrations of H2SO4 and HNO3, with HNO3 proving to have the faster reaction kinetics. Porous powders were analyzed by differential scanning calorimetry to observe crystallization behavior. The Microstructure of milled powder and dissolved powder was analyzed by scanning electron microscope. To check for residual in the dissolved powder after dissolution, energy dispersive X-ray spectroscory and elemental mapping was conducted. It was confirmed that the MG/brass composite powder dissolved in 10% HNO3 produced a porous MG granule with a relatively high specific surface area of 19.60 m2/g. This proved to be the optimum dissolution condition in which both a porous internal granule structure and amorphous phase were maintained. Consequently, porous MG granules were effectively fabricated and applications of such structures can be expanded.
-
Citations
Citations to this article as recorded by- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
TOP
 KPMI
KPMI


 First
First Prev
Prev


