Search
- Page Path
- HOME > Search
- [Korean]
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(4):324-328. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00199

- 862 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - This study analyzed the influence of ball size and process control agents on the refinement and dehydrogenation behavior of TiH2 powder. Powders milled using ZrO2 balls with diameters of 0.1 mm, 0.3 mm, and 0.3+0.5+1 mm exhibited a bimodal particle size distribution, of which the first mode had the smallest size of 0.23 μm for the 0.3 mm balls. Using ethanol and/or stearic acid as process control agents was effective in particle refinement. Thermogravimetric analysis showed that dehydrogenation of the milled powder started at a relatively low temperature compared to the raw powder, which is interpreted to have resulted from a decrease in particle size and an increase in defects. The dehydrogenation kinetics of the TiH2 powder were evaluated by the magnitude of peak shift with heating rates using thermogravimetric analysis. The activation energy of the dehydrogenation reaction, calculated from the slope of the Kissinger plot, was measured to be 228.6 kJ/mol for the raw powder and 194.5 kJ/mol for the milled powder. TEM analysis revealed that both the milled and dehydrogenated powders showed an angular shape with a size of about 200 nm.
- [Korean]
- Effect of Ball Milling Conditions on the Microstructure and Dehydrogenation Behavior of TiH2 Powder
- Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
- J Powder Mater. 2024;31(2):132-136. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00001

- 1,880 View
- 35 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - This study investigated the effects of revolution speed and ball size in planetary milling on the microstructure and dehydrogenation behavior of TiH2 powder. The particle size analysis showed that the large particles present in the raw powder were effectively refined as the revolution speed increased, and when milled at 500 rpm, the median particle size was 1.47 m. Milling with a mixture of balls of two or three sizes was more effective in refining the raw powder than milling with balls of a single size. A mixture of 3-mm and 5-mm-diameter balls was the optimal condition for particle refinement, and the measured median particle size was 0.71 m. The dependence of particle size on revolution speed and ball size was explained by changes in input energy and the number of contact points of the balls. In the milled powder, the endothermic peak measured using differential thermal analysis was observed at a relatively low temperature. This finding was interpreted as the activation of a dehydrogenation reaction, mainly due to the increase in the specific surface area and the concentration of lattice defects.
-
Citations
Citations to this article as recorded by- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
Ji Young Kim, Eui Seon Lee, Ji Won Choi, Youngmin Kim, Sung-Tag Oh
Journal of Powder Materials.2024; 31(4): 324. CrossRef
- Preparation and Microstructural Characteristics of Ti Nanopowder by Ball Milling and Dehydrogenation of TiH2 Powder
- [Korean]
- The Fabrication of Cobalt Nanopowder by Sonochemical Polyol Synthesis of Cobalt Hydroxide and Magnetic Separation Method
- Jong Min Byun, Myoung Hwan Choi, Chang Min Shim, Ji Young Kim, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(1):39-45. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.39

- 679 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF In this study, cobalt nanopowder is fabricated by sonochemical polyol synthesis and magnetic separation method. First, sonochemical polyol synthesis is carried out at 220°C for up to 120 minutes in diethylene glycol (C4H10O3). As a result, when sonochemical polyol synthesis is performed for 50 minutes, most of the cobalt precursor (Co(OH)2) is reduced to spherical cobalt nanopowder of approximately 100 nm. In particular, aggregation and growth of cobalt particles are effectively suppressed as compared to common polyol synthesis. Furthermore, in order to obtain finer cobalt nanopowder, magnetic separation method using magnetic property of cobalt is introduced at an early reduction stage of sonochemical polyol synthesis when cobalt and cobalt precursor coexist. Finally, spherical cobalt nanopowder having an average particle size of 22 nm is successfully separated.
- [Korean]
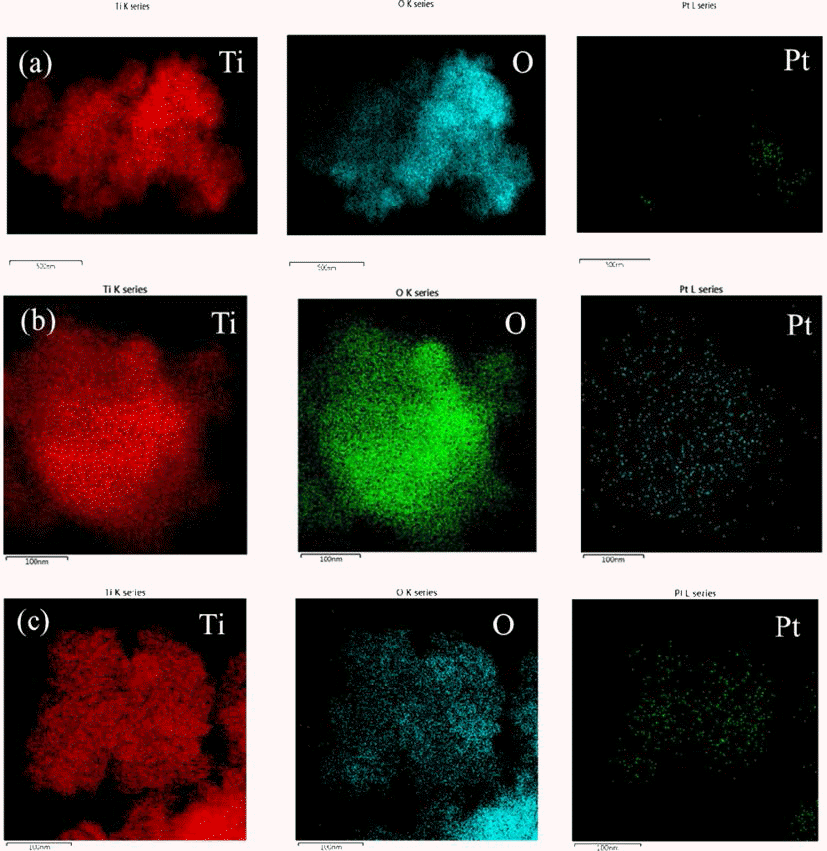
- Synthesis of Pt@TiO2 Nano-composite via Photochemical Reduction Method
- Ji Young Kim, Jong Min Byun, Jin Woo Kim, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(2):119-123. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.119
- 655 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Pt has been widely used as catalyst for fuel cell and exhausted gas clean systems due to its high catalytic activity. Recently, there have been researches on fabricating composite materials of Pt as a method of reducing the amount of Pt due to its high price. One of the approaches for saving Pt used as catalyst is a core shell structure consisting of Pt layer on the core of the non-noble metal. In this study, the synthesis of Pt shell was conducted on the surface of TiO2 particle, a non-noble material, by applying ultraviolet (UV) irradiation. Anatase TiO2 particles with the average size of 20~30 nm were immersed in the ethanol dissolved with Pt precursor of H2PtCl6∙6H2O and exposed to UV irradiation with the wavelength of 365 nm. It was confirmed that Pt nano-particles were formed on the surface of TiO2 particles by photochemical reduction of Pt ion from the solution. The morphology of the synthesized Pt@TiO2 nano-composite was examined by TEM (Transmission Electron Microscopy).
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
Hyeong-Chul Kim, Jae-Kil Han
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 33. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


