Search
- Page Path
- HOME > Search
- [Korean]
- Effects of the Content of MgO Additive and Sintering Temperature on the Densification of Alumina Insulator
- Ri Joo Kim, Han Gyeol Jeong, Ye Ji Son, Sang Ki Ko, Hyun Seon Hong
- J Powder Mater. 2023;30(3):249-254. Published online June 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.3.249
- 1,427 View
- 23 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF The influence of MgO addition on the densification and microstructure of alumina (Al2O3) was studied. Compacted alumina specimens were manufactured using ball-milling and one-directional pressing followed by sintering at temperatures below 1700oC. Relative density, shrinkage, hardness, and microstructure were investigated using analytical tools such as FE-SEM, EDS, and XRD. When the MgO was added up to 5.0 wt% and sintered at 1500°C and 1600°C, the relative density exhibited an average value of 97% or more at both temperatures. The maximum density of 99.2% was with the addition of 0.5 wt% MgO at 1500°C. Meanwhile, the specimens showed significantly lower density values when sintered at 1400°C than at 1500°C and 1600°C owing to the relatively low sintering temperature. The hardness and shrinkage data also showed a similar trend in the change in density, implying that the addition of approximately 0.5 wt% MgO can promote the densification of Al2O3. Studying the microstructure confirmed the uniformity of the sintered alumina. These results can be used as basic compositional data for the development of MgOcontaining alumina as high-dielectric insulators.
-
Citations
Citations to this article as recorded by- Hardness of Ultra-High-Density Alumina Fabricated using the Aerodynamic Levitation Process
Ye-Ji Son, Dong-Wook Kim, Seung-Wook Kim, Hyo-Min Kim, Hui-Woong Kang, Min-Yeong Ha, Dae-Yong Jeong
Korean Journal of Materials Research.2025; 35(9): 436. CrossRef
- Hardness of Ultra-High-Density Alumina Fabricated using the Aerodynamic Levitation Process
- [Korean]
- Effect of H2SO4 and Reaction Time on Synthesis of 5Mg(OH)2∙MgSO4∙3H2O Whiskers using Hydrothermal Reaction
- Areum Choi, Nuri Oh, YooJin Kim
- J Korean Powder Metall Inst. 2020;27(5):401-405. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.401

- 1,035 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Magnesium hydroxide sulfate hydrate (MHSH) whiskers were synthesized via a hydrothermal reaction by using MgO as the reactant as well as the acid solution. The effects of the H2SO4 amount and reaction time at the same temperature were studied. In general, MHSH whiskers were prepared using MgSO4 in aqueous ammonia. In this work, to reduce the formation of impurities and increase the purity of MHSH, we employed a synthesis technique that did not require the addition of a basic solution. Furthermore, the pH value, which was controlled by the H2SO4 amount, acted as an important factor for the formation of high-purity MHSH. MgO was used as the raw material because it easily reacts in water and forms Mg+ and MgOH+ ions that bind with SO4 2- ions to produce MHSH. Their morphologies and structures were determined using X-ray diffraction (XRD) and scanning electron microscopy (SEM).
-
Citations
Citations to this article as recorded by- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
Journal of Powder Materials.2025; 32(5): 399. CrossRef - Study of SiO2 coating and carboxylic surface-modification on Mg-based inorganic fiber by one-step reflux reaction
Minsol Park, Areum Choi, Seiki Kim, Wooyoung Shim, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(6): 869. CrossRef - Effect of sulfate ion on synthesis of 5 Mg(OH)2·MgSO4·3H2O whiskers using non-hydrothermal method with acid catalyst
Areum Choi, Nuri Oh, YooJin Kim
Journal of the Korean Ceramic Society.2022; 59(2): 224. CrossRef
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
- [English]
- Fabrication and Characterization of Thermal Battery using Porous MgO Separator Infiltrated with Li based Molten Salts
- Kyungho Kim, Sungmin Lee, Chae-Nam Im, Seung-Ho Kang, Hae-Won Cheong, Yoonsoo Han
- J Korean Powder Metall Inst. 2017;24(5):364-369. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.364
- 836 View
- 9 Download
-
 Abstract
Abstract
 PDF
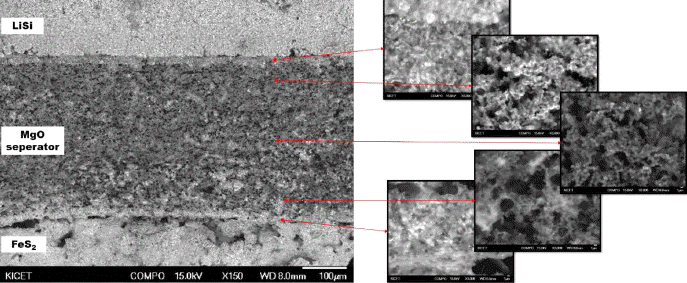
PDF Ceramic powder, such as MgO, is added as a binder to prepare the green compacts of molten salts of an electrolyte for a thermal battery. Despite the addition of a binder, when the thickness of the electrolyte decreases to improve the battery performance, the problem with the unintentional short circuit between the anode and cathode still remains. To improve the current powder molding method, a new type of electrolyte separator with porous MgO preforms is prepared and characteristics of the thermal battery are evaluated. A Spherical PMMA polymer powder is added as a pore-forming agent in the MgO powder, and an organic binder is used to prepare slurry appropriate for tape casting. A porous MgO preform with 300 μm thickness is prepared through a binder burnout and sintering process. The particle size of the starting MgO powder has an effect, not on the porosity of the porous MgO preform, but on the battery characteristics. The porosity of the porous MgO preforms is controlled from 60 to 75% using a pore-forming agent. The batteries prepared using various porosities of preforms show a performance equal to or higher than that of the pellet-shaped battery prepared by the conventional powder molding method.
TOP
 KPMI
KPMI


 First
First Prev
Prev


