Search
- Page Path
- HOME > Search
- [Korean]
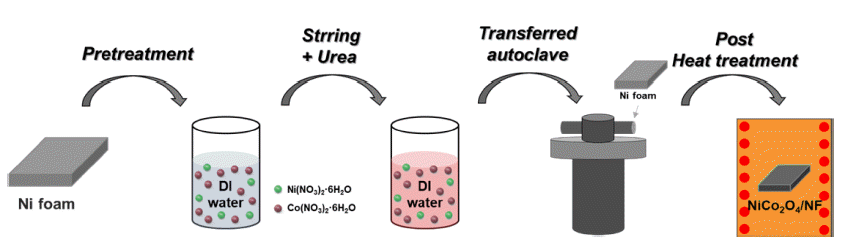
- Fabrication and Characterization of NiCo2O4/Ni Foam Electrode for Oxygen Evolution Reaction in Alkaline Water Splitting
- Minsol Kwon, Jaeseong Go, Yesol Lee, Sungmin Lee, Jisu Yu, Hyowon Lee, Sung Ho Song, Dongju Lee
- J Powder Mater. 2022;29(5):411-417. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.411
- 1,122 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF Environmental issues such as global warming due to fossil fuel use are now major worldwide concerns, and interest in renewable and clean energy is growing. Of the various types of renewable energy, green hydrogen energy has recently attracted attention because of its eco-friendly and high-energy density. Electrochemical water splitting is considered a pollution-free means of producing clean hydrogen and oxygen and in large quantities. The development of non-noble electrocatalysts with low cost and high performance in water splitting has also attracted considerable attention. In this study, we successfully synthesized a NiCo2O4/NF electrode for an oxygen evolution reaction in alkaline water splitting using a hydrothermal method, which was followed by post-heat treatment. The effects of heat treatment on the electrochemical performance of the electrodes were evaluated under different heat-treatment conditions. The optimized NCO/NF-300 electrode showed an overpotential of 416 mV at a high current density of 50 mA/cm2 and a low Tafel slope (49.06 mV dec-1). It also showed excellent stability (due to the large surface area) and the lowest charge transfer resistance (12.59 Ω). The results suggested that our noble-metal free electrodes have great potential for use in developing alkaline electrolysis systems.
- [Korean]
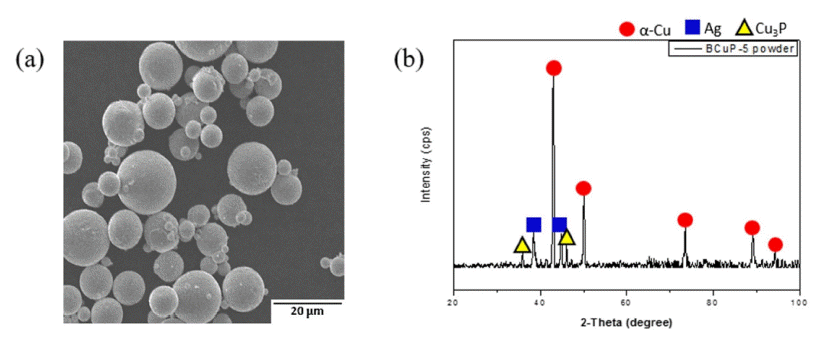
- Effect of Post Heat Treatment on the Microstructure and Mechanical Properties of BCuP-5 Filler Metal Coating Layers Fabricated by High Velocity Oxygen Fuel Thermal Spray Process on Ag Substrate
- So-Yeon Park, Seong-June Youn, Jae-Sung Park, Kee-Ahn Lee
- J Powder Mater. 2022;29(4):283-290. Published online August 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.4.283

- 1,169 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF A Cu-15Ag-5P filler metal (BCuP-5) is fabricated on a Ag substrate using a high-velocity oxygen fuel (HVOF) thermal spray process, followed by post-heat treatment (300°C for 1 h and 400°C for 1 h) of the HVOF coating layers to control its microstructure and mechanical properties. Additionally, the microstructure and mechanical properties are evaluated according to the post-heat treatment conditions. The porosity of the heat-treated coating layers are significantly reduced to less than half those of the as-sprayed coating layer, and the pore shape changes to a spherical shape. The constituent phases of the coating layers are Cu, Ag, and Cu-Ag-Cu3P eutectic, which is identical to the initial powder feedstock. A more uniform microstructure is obtained as the heat-treatment temperature increases. The hardness of the coating layer is 154.6 Hv (as-sprayed), 161.2 Hv (300°C for 1 h), and 167.0 Hv (400°C for 1 h), which increases with increasing heat-treatment temperature, and is 2.35 times higher than that of the conventional cast alloy. As a result of the pull-out test, loss or separation of the coating layer rarely occurs in the heat-treated coating layer.
-
Citations
Citations to this article as recorded by- Evaluation and Prediction of Mechanical Properties According to Welding Methods of Ni 825/A516-70N Clad Plates
Cheolhong Hwang, Jeongseok Oh, Jini Park, Myungwoo Joe, Sunhwan Kim, Kyunghoon Yoo, Sungwoong Kim, Youngjoo Kim, Sangyeob Lee, Joonsik Park
Korean Journal of Metals and Materials.2024; 62(11): 844. CrossRef
- Evaluation and Prediction of Mechanical Properties According to Welding Methods of Ni 825/A516-70N Clad Plates
- [Korean]
- Fabrication, Microstructure and Adhesion Properties of BCuP-5 Filler Metal/Ag Plate Clad Material by Using High Velocity Oxygen Fuel Thermal Spray Process
- Yeun A Joo, Yong-Hoon Cho, Jae-Sung Park, Kee-Ahn Lee
- J Powder Mater. 2022;29(3):226-232. Published online June 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.3.226
- 936 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF In this study, a new manufacturing process for a multilayer-clad electrical contact material is suggested. A thin and dense BCuP-5 (Cu-15Ag-5P filler metal) coating layer is fabricated on a Ag plate using a high-velocity oxygen-fuel (HVOF) process. Subsequently, the microstructure and bonding properties of the HVOF BCuP-5 coating layer are evaluated. The thickness of the HVOF BCuP-5 coating layer is determined as 34.8 μm, and the surface fluctuation is measured as approximately 3.2 μm. The microstructure of the coating layer is composed of Cu, Ag, and Cu-Ag-Cu3P ternary eutectic phases, similar to the initial BCuP-5 powder feedstock. The average hardness of the coating layer is 154.6 HV, which is confirmed to be higher than that of the conventional BCuP-5 alloy. The pull-off strength of the Ag/BCup-5 layer is determined as 21.6 MPa. Thus, the possibility of manufacturing a multilayer-clad electrical contact material using the HVOF process is also discussed.
- [Korean]
- Evaluation of Oxygen Reduction and Surface Chemical State of Ti-48Al-2Cr-2Nb Powder by Ca Vapor
- Taeheon Kim, Hanjung Kwon, Jae-Won Lim
- J Korean Powder Metall Inst. 2021;28(1):31-37. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.31

- 1,111 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF This study explores reducing the oxygen content of a commercial Ti-48Al-2Cr-2Nb powder to less than 400 ppm by deoxidation in the solid state (DOSS) using Ca vapor, and investigates the effect of Ca vapor on the surface chemical state. As the deoxidation temperature increases, the oxygen concentration of the Ti-48Al-2Cr-2Nb powder decreases, achieving a low value of 745 ppm at 1100°C. When the deoxidation time is increased to 2 h, the oxygen concentration decreases to 320pp m at 1100°C, and the oxygen reduction rate is approximately 78% compared to that of the raw material. The deoxidized Ti-48Al-2Cr-2nb powder maintains a spherical shape, but the surface shape changes slightly owing to the reaction of Ca and Al. The oxidation state of Ti and Al on the surface of the Ti-48Al-2Cr-2Nb powder corresponds to a mixture of TiO2 and Al2O3. As a result, the peaks of metallic Ti and Ti suboxide intensify as TiO2 and Al2O3 in the surface oxide layer are reduced by Ca vapor deposition
-
Citations
Citations to this article as recorded by- Production of spherical TiAl alloy powder by copper-assisted spheroidization
Jin Qian, Bo Yin, Dashun Dong, Geng Wei, Ming Shi, Shaolong Tang
Journal of Materials Research and Technology.2023; 25: 1860. CrossRef - Ca-Mg Multiple Deoxidation of Ti-50Al-2Cr-2Nb Intermetallic Compound Powder for Additive Manufacturing
Seongjae Cho, Taeheon Kim, Jae-Won Lim
ECS Journal of Solid State Science and Technology.2022; 11(4): 045008. CrossRef
- Production of spherical TiAl alloy powder by copper-assisted spheroidization
- [Korean]
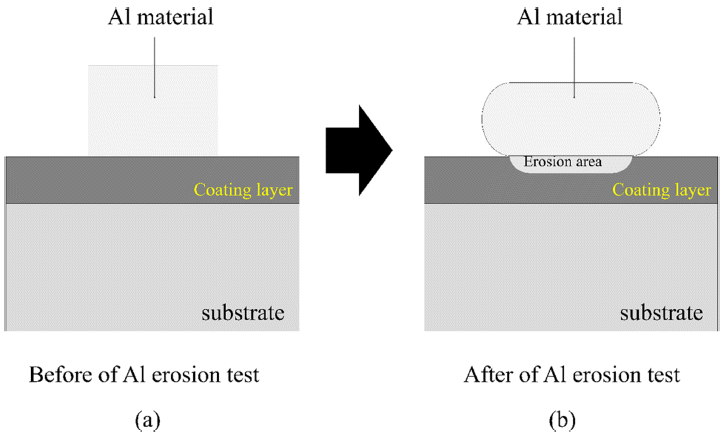
- Microstructure and Liquid Al Erosion Property of Tribaloy T-800 Coating Material Manufactured by Laser Cladding Process
- Kyoung-Wook Kim, Gi-Su Ham, Sun-Hong Park, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2020;27(3):210-218. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.210
- 580 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF A T-800 (Co-Mo-Cr) coating material is fabricated using Co-Mo-Cr powder feedstock and laser cladding. The microstructure and melted Al erosion properties of the laser-cladded T-800 coating material are investigated. The Al erosion properties of the HVOF-sprayed MoB-CoCr and bulk T-800 material are also examined and compared with the laser-cladded T-800 coating material. Co and lave phases (Co2MoCr and Co3Mo2Si) are detected in both the lasercladded T-800 coating and the bulk T-800 materials. However, the sizes of the lave phases are measured as 7.9 μm and 60.6 μm for the laser-cladded and bulk T-800 materials, respectively. After the Al erosion tests, the erosion layer thicknesses of the three materials are measured as 91.50 μm (HVOF MoB-CoCr coating), 204.83 μm (laser cladded T-800), and 226.33 μm (bulk T-800). In the HVOF MoB-CoCr coating material, coarse cracks and delamination of the coating layer are observed. On the other hand, no cracks or local delamination of the coating layer are detected in the laser T-800 material even after the Al erosion test. Based on the above results, the authors discuss the appropriate material and process that could replace conventional bulk T-800 materials used as molten Al pots.
- [Korean]
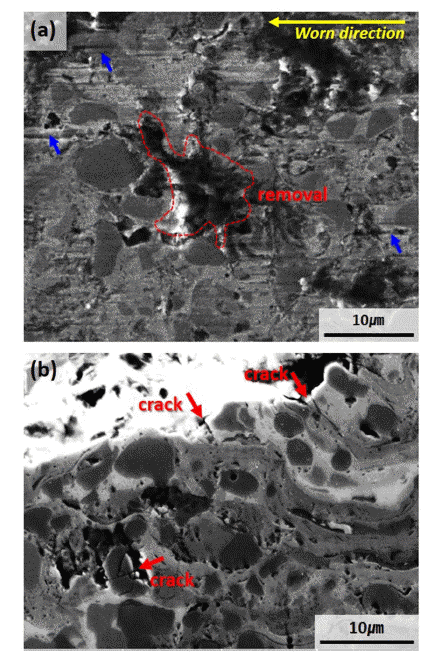
- Microstructural and Wear Properties of WC-based and Cr3C2-based Cermet Coating Materials Manufactured with High Velocity Oxygen Fuel Process
- Yeon-Ji Kang, Gi-Su Ham, Hyung-Jun Kim, Sang-Hoon Yoon, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2018;25(5):408-414. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.408
- 890 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF This study investigates the microstructure and wear properties of cermet (ceramic + metal) coating materials manufactured using high velocity oxygen fuel (HVOF) process. Three types of HVOF coating layers are formed by depositing WC-12Co, WC-20Cr-7Ni, and Cr3C2-20NiCr (wt.%) powders on S45C steel substrate. The porosities of the coating layers are 1 ± 0.5% for all three specimens. Microstructural analysis confirms the formation of second carbide phases of W2C, Co6W6C, and Cr7C3 owing to decarburizing of WC phases on WC-based coating layers. In the case of WC-12Co coating, which has a high ratio of W2C phase with high brittleness, the interface property between the carbide and the metal binder slightly decreases. In the Cr3C2-20CrNi coating layer, decarburizing almost does not occur, but fine cavities exist between the splats. The wear loss occurs in the descending order of Cr3C2-20NiCr, WC-12Co, and WC-20Cr-7Ni, where WC-20Cr-7Ni achieves the highest wear resistance property. It can be inferred that the ratio of the carbide and the binding properties between carbide–binder and binder–binder in a cermet coating material manufactured with HVOF as the primary factors determine the wear properties of the cermet coating material.
-
Citations
Citations to this article as recorded by- Tribological Behavior Analysis of WC-Ni-Cr + Cr3C2 and WC-Ni-Cr + YSZ Coatings Sprayed by HVOF
Tae-Jun Park, Gye-Won Lee, Yoon-Suk Oh
journal of Korean Powder Metallurgy Institute.2023; 30(5): 415. CrossRef - Effects of different HVOF thermal sprayed cermet coatings on tensile and fatigue properties of AISI 1045 steel
Gi-Su Ham, R. Kreethi, Hyung-jun Kim, Sang-hoon Yoon, Kee-Ahn Lee
Journal of Materials Research and Technology.2021; 15: 6647. CrossRef
- Tribological Behavior Analysis of WC-Ni-Cr + Cr3C2 and WC-Ni-Cr + YSZ Coatings Sprayed by HVOF
- [Korean]
- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
- Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2017;24(2):96-101. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.96
- 922 View
- 3 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
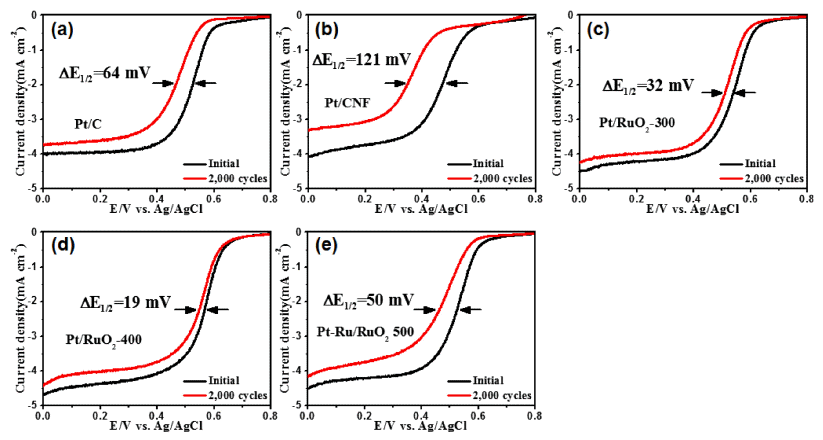
PDF Well-dispersed platinum catalysts on ruthenium oxide nanofiber supports are fabricated using electrospinning, post-calcination, and reduction methods. To obtain the well-dispersed platinum catalysts, the surface of the nanofiber supports is modified using post-calcination. The structures, morphologies, crystal structures, chemical bonding energies, and electrochemical performance of the catalysts are investigated. The optimized catalysts show well-dispersed platinum nanoparticles (1-2 nm) on the nanofiber supports as well as a uniform network structure. In particular, the well-dispersed platinum catalysts on the ruthenium oxide nanofiber supports display excellent catalytic activity for oxygen reduction reactions with a half-wave potential (E1/2) of 0.57 V and outstanding long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 19 mV. The enhanced electrochemical performance for oxygen reduction reactions results from the well-dispersed platinum catalysts and unique nanofiber supports.
-
Citations
Citations to this article as recorded by- Fabrication of Porous Electrodes for Zinc-Ion Supercapacitors with Improved Energy Storage Performance
Geon-Hyoung An
Korean Journal of Materials Research.2019; 29(8): 505. CrossRef - Surface tailoring of zinc electrodes for energy storage devices with high-energy densities and long cycle life
Geon-Hyoung An, SeungNam Cha, Jung Inn Sohn
Applied Surface Science.2019; 467-468: 1157. CrossRef - Synthesis of Nitrogen Doped Protein Based Carbon as Pt Catalysts Supports for Oxygen Reduction Reaction
Young-geun Lee, Geon-hyeong An, Hyo-Jin Ahn
Korean Journal of Materials Research.2018; 28(3): 182. CrossRef
- Fabrication of Porous Electrodes for Zinc-Ion Supercapacitors with Improved Energy Storage Performance
- [Korean]
- Synthesis of Nitrogen-doped Carbon Nanofibers for Oxygen Reduction Reaction
- Geon-Hyoung An, Eun-Hwan Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(6):420-425. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.420

- 647 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF N-doped carbon nanofibers as catalysts for oxygen-reduction reactions are synthesized using electrospinning and carbonization. Their morphologies, structures, chemical bonding states, and electrochemical performance are characterized. The optimized N-doped carbon nanofibers exhibit graphitization of carbon nanofibers and an increased nitrogen doping as well as a uniform network structure. In particular, the optimized N-doped carbon nanofibers show outstanding catalytic activity for oxygen-reduction reactions, such as a half-wave potential (E1/2) of 0.43 V, kinetic limiting current density of 6.2 mA cm-2, electron reduction pathways (n = 3.1), and excellent long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 13 mV. The improvement in the electrochemical performance results from the synergistic effect of the graphitization of carbon nanofibers and the increased amount of nitrogen doping.
TOP
 KPMI
KPMI


 First
First Prev
Prev


