Search
- Page Path
- HOME > Search
- [Korean]
- Filling and Wiping Properties of Silver Nano Paste in Trench Layer of Metal Mesh Type Transparent Conducting Electrode Films for Touch Screen Panel Application
- Gi-Dong Kim, Hyun-Min Nam, Sangsun Yang, Lee-Soon Park, Su-Yong Nam
- J Korean Powder Metall Inst. 2017;24(6):464-471. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.464

- 1,140 View
- 8 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF A metal mesh TCE film is fabricated using a series of processes such as UV imprinting of a transparent trench pattern (with a width of 2-5 μm) onto a PET film, filling it with silver paste, wiping of the surface, and heatcuring the silver paste. In this work nanosized (40-50 nm) silver particles are synthesized and mixed with submicron (250-300 nm)-sized silver particles to prepare silver paste for the fabrication of metal mesh-type TCE films. The filling of these silver pastes into the patterned trench layer is examined using a specially designed filling machine and the rheological testing of the silver pastes. The wiping of the trench layer surface to remove any residual silver paste or particles is tested with various mixture solvents, and ethyl cellosolve acetate (ECA):DI water = 90:10 wt% is found to give the best result. The silver paste with 40-50 nm Ag:250-300 nm Ag in a 10:90 wt% mixture gives the highest electrical conductance. The metal mesh TCE film obtained with this silver paste in an optimized process exhibits a light transmittance of 90.4% and haze at 1.2%, which is suitable for TSP application.
-
Citations
Citations to this article as recorded by- Silver and epoxy binder-based printed electrodes and the effect of silver nanoparticles on stretchability
Suk Hun Hyun, Se-Hoon Park, Sung-Hoon Choa, Hyun Jin Nam, Heejoon Ahn
Journal of Materials Science: Materials in Electronics.2019; 30(19): 17591. CrossRef - Electro-mechanical Properties of Stretchable Ag Paste by the Difference of Ag Particles
Sun-Young Kang, Min-Young Park, Dong-Young Jang
Journal of the Korean Society of Manufacturing Technology Engineers.2019; 28(3): 188. CrossRef
- Silver and epoxy binder-based printed electrodes and the effect of silver nanoparticles on stretchability
- [Korean]
- A Study on the Improvement of Storage Stability of Solder Paste Using Multiple size of solder Powder
- Chan-Kyu Lim, Bo-Suk Gyun, Min-Jung Son, Inyoung Kim, Sangsun Yang, Su-Yong Nam
- J Korean Powder Metall Inst. 2017;24(5):395-399. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.395

- 1,192 View
- 9 Download
-
 Abstract
Abstract
 PDF
PDF Solder paste is widely used as a conductive adhesive in the electronics industry. In this paper, nano and microsized mixed lead-free solder powder (Sn-Ag-Cu) is used to manufacture solder paste. The purpose of this paper is to improve the storage stability using different types of solvents that are used in fabricating the solder paste. If a solvent of sole acetate is used, the nano sized solder powder and organic acid react and form a Sn-Ag-Cu malonate. These formed malonates create fatty acid soaps. The fatty acid soaps absorb the solvents and while the viscosity of the solder paste rises, the storage stability and reliability decrease. When ethylene glycol, a dihydric alcohol, is used the fatty acid soaps and ethylene glycol react, preventing the further creation of the fatty acid soaps. The prevention of gelation results in an improvement in the solder paste storage ability.
- [Korean]
- Synthesis of DyF3 paste and Magnetic Properties of GBDPed Nd-Fe-B Magnets
- Kwang-Won Jeon, Hee-Ryoung Cha, Jung-Goo Lee
- J Korean Powder Metall Inst. 2016;23(6):437-441. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.437
- 607 View
- 2 Download
-
 Abstract
Abstract
 PDF
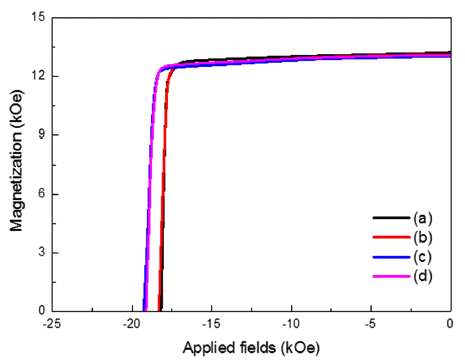
PDF Recently, the grain boundary diffusion process (GBDP), involving heavy rare-earth elements such as Dy and Tb, has been widely used to enhance the coercivity of Nd-Fe-B permanent magnets. For example, a Dy compound is coated onto the surface of Nd-Fe-B sintered magnets, and then the magnets are heat treated. Subsequently, Dy diffuses into the grain boundaries of Nd-Fe-B magnets, forming Dy-Fe-B or Nd-Dy-Fe-B. The dip-coating process is also used widely instead of the GBDP. However, it is quite hard to control the thickness uniformity using dip coating. In this study, first, a DyF3 paste is fabricated using DyF3 powder. Subsequently, the fabricated DyF3 paste is homogeneously coated onto the surface of a Nd-Fe-B sintered magnet. The magnet is then subjected to GBDP to enhance its coercivity. The weight ratio of binder and DyF3 powder is controlled, and we find that the coercivity enhances with decreasing binder content. In addition, the maximum coercivity is obtained with the paste containing 70 wt% of DyF3 powder.
- [Korean]
- The Preparation of Dye-Sensitized Solar Cell Paste Used the Peroxo Titanium Complex and Characteristics by Annealing Temperature
- Hyunsu Park, Soyeong Joo, Joon-Phil Choi, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2015;22(6):396-402. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.396
- 999 View
- 6 Download
- 5 Citations
-
 Abstract
Abstract
 PDF
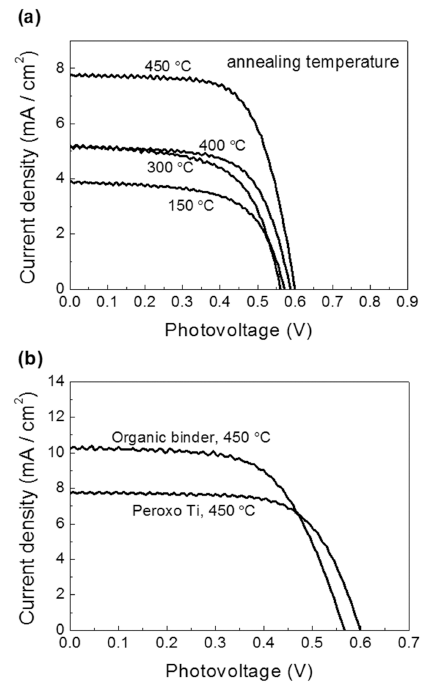
PDF The organic binder-free paste for dye-sensitized solar cell (DSSC) has been investigated using peroxo titanium complex. The crystal structure of TiO2 nanoparticles, morphology of TiO2 film and electrical properties are analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Electrochemical Impedance Spectra (EIS), and solar simulator. The synthesized TiO2 nanopowders by the peroxo titanium complex at 150, 300, 400°C, and 450°C have anatase phase and average crystal sizes are calculated to be 4.2, 13.7, 16.9, and 20.9 nm, respectively. The DSSC prepared by the peroxo titanium complex binder have higher Voc and lower Jsc values than that of the organic binder. It can be attributed to improvement of sintering properties of TCO/TiO2 and TiO2/TiO2 interface and to formation of agglomerate by the nanoparticles. As a result, we have investigated the organic binder-free paste and 3.178% conversion efficiency of the DSSC at 450°C.
-
Citations
Citations to this article as recorded by- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
Won Jin Kim, Jinho Lee, Ryun Na Kim, Donghee Lee, Woo-Byoung Kim
Korean Journal of Materials Research.2024; 34(3): 152. CrossRef - Development of eco-friendly thin film manufacturing process using poeroxo titanium complex solution and potential for resistive random access memory
Jinho Lee, Ryun Na Kim, Kee-Ryung Park, Woo-Byoung Kim
Applied Surface Science.2021; 562: 150170. CrossRef - Preparation of ultra-thin TiO2 shell by peroxo titanium complex (PTC) solution-based green surface modification, and photocatalytic activity of homo-core/shell TiO2
Jinho Lee, Jiyong Hwang, Hyunsu Park, Tohru Sekino, Woo-Byoung Kim
Applied Surface Science.2021; 540: 148399. CrossRef - Effects of Annealing Temperature on the Crystal Structure, Morphology, and Optical Properties of Peroxo-Titanate Nanotubes Prepared by Peroxo-Titanium Complex Ion
Hyunsu Park, Tomoyo Goto, Sunghun Cho, Soo Wohn Lee, Masato Kakihana, Tohru Sekino
Nanomaterials.2020; 10(7): 1331. CrossRef - Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(5): 353. CrossRef
- Development of Eco-Friendly Ag Embedded Peroxo Titanium Complex Solution Based Thin Film and Electrical Behaviors of Resistive Random Access Memory
TOP
 KPMI
KPMI


 First
First Prev
Prev


