Search
- Page Path
- HOME > Search
- [Korean]
- Optical Properties of Spherical YAG:Ce3+ Phosphor Powders Synthesized by Atmospheric Plasma Spraying Method Appling PVA Solution Route and Domestic Aluminium Oxide Seed
- Yong-Hyeon Kim, Sang-Jin Lee
- J Powder Mater. 2023;30(5):424-430. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.424
- 957 View
- 3 Download
-
 Abstract
Abstract
 PDF
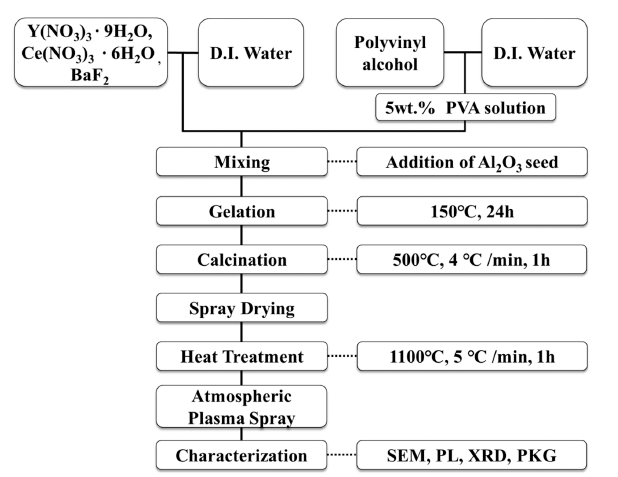
PDF YAG phosphor powders were fabricated by the atmospheric plasma spraying method with the spray-dried spherical YAG precursor. The YAG precursor slurry for the spray drying process was prepared by the PVA solution chemical processing utilizing a domestic easy-sintered aluminum oxide (Al2O3) powder as a seed. The homogenous and viscous slurry resulted in dense granules, not hollow or porous particles. The synthesized phosphor powders demonstrated a stable YAG phase, and excellent fluorescence properties of approximately 115% compared with commercial YAG:Ce3+ powder. The microstructure of the phosphor powder had a perfect spherical shape and an average particle s ize of a pprox imately 30 μm. As a r esult of t he PKG t est of t he YAG p hosphor p owder, t he s ynthesized phosphor powders exhibited an outstanding luminous intensity, and a peak wavelength was observed at 531 nm.
- [Korean]
- Fluorescent Nanoparticles: Synthesis and Applications
- Y. K. Kim, B. K. Song, J. G. Lee, Y. K. Baek
- J Korean Powder Metall Inst. 2020;27(2):154-163. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.154
- 2,905 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
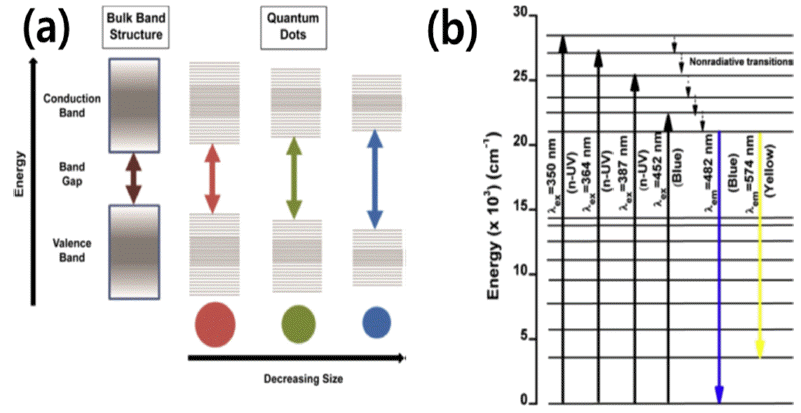
PDF Fluorescent nanoparticles are characterized by their unique properties such as luminescence, optical transparency, and sensitivity to various chemical environments. For example, semiconductor nanocrystals (quantum dots), which are nanophosphors doped with transition metal or rare earth ions, can be classified as fluorescent nanoparticles. Tuning their optical and physico-chemical properties can be carried out by considering and taking advantage of nanoscale effects. For instance, quantum confinement causes a much higher fluorescence with nanoparticles than with their bulk counterparts. Recently, various types of fluorescent nanoparticles have been synthesized to extend their applications to other fields. In this study, State-of-the-art fluorescent nanoparticles are reviewed with emphasis on their analytical and anti-counterfeiting applications and synthesis processes. Moreover, the fundamental principles behind the exceptional properties of fluorescent nanoparticles are discussed.
-
Citations
Citations to this article as recorded by- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
Ji-Won Heo, A-Young Sung
Korean Journal of Materials Research.2022; 32(4): 193. CrossRef
- Preparation and Analysis of High Functional Silicone Hydrogel Lens Containing Metal Oxide Nanoparticles by Photopolymerizaion
- [Korean]
- Photoluminescence Enhancement of Y2O3:Eu3+ Red Phosphor Prepared by Spray Pyrolysis using Aliovalent Cation Substitution and Organic Additives
- Byeong Ho Min, Kyeong Youl Jung
- J Korean Powder Metall Inst. 2020;27(2):146-153. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.146
- 690 View
- 5 Download
-
 Abstract
Abstract
 PDF
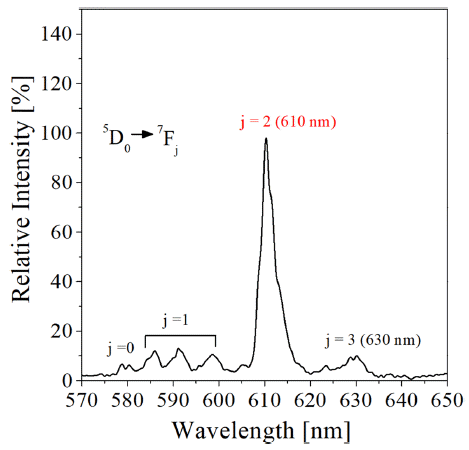
PDF The co-doping effect of aliovalent metal ions such as Mg2+, Ca2+, Sr2+, Ba2+, and Zn2+ on the photoluminescence of the Y2O3:Eu3+ red phosphor, prepared by spray pyrolysis, is analyzed. Mg2+ metal doping is found to be helpful for enhancing the luminescence of Y2O3:Eu3+. When comparing the luminescence intensity at the optimum doping level of each Mg2+ ion, the emission enhancement shows the order of Zn2+ ≈ Ba2+ > Ca2+ > Sr3+> Mg2+. The highest emission occurs when doping approximately 1.3% Zn2+, which is approximately 127% of the luminescence intensity of pure Y2O3:Eu3+. The highest emission was about 127% of the luminescence intensity of pure Y2O3:Eu3+ when doping about 1.3% Zn2+. It is determined that the reason (Y, M)2O3:Eu3+ has improved luminescence compared to that of Y2O3:Eu3+ is because the crystallinity of the matrix is improved and the non-luminous defects are reduced, even though local lattice strain is formed by the doping of aliovalent metal. Further improvement of the luminescence is achieved while reducing the particle size by using Li2CO3 as a flux with organic additives.
- [Korean]
- Effect of Phosphorus Addition on Microstructure and Mechanical Properties of Sintered Low Alloy Steel
- Yoo-Young Kim, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2020;27(1):31-36. Published online February 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.1.31

- 798 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Phosphorus is an element that plays many important roles in powder metallurgy as an alloy element. The purpose of this study is to investigate the influence of phosphorus addition on the microstructures and mechanical properties of sintered low-alloy steel. The sintered low-alloy steels Fe-0.6%C-3.89%Ni-1.95%Cu-1.40%Mo-xP (x=0, 0.05, 0.10, 0.15, 0.20%) were manufactured by compacting at 700 MPa, sintering in H2-N2 at 1260 °C, rapid cooling, and low-temperature tempering in Ar at 160 °C. The microstructure, pore, density, hardness, and transverse rupture strength (TRS) of the sintered low-alloy steels were evaluated. The hardness increased as the phosphorus content increased, whereas the density and TRS showed maximum values when the content of P was 0.05%. Based on microstructure observation, the phase of the microstructure changed from bainite to martensite as the content of phosphorus is increased. Hence, the most appropriate addition of phosphorus in this study was 0.05%.
-
Citations
Citations to this article as recorded by- A new strategy for metal additive manufacturing using an economical water-atomized iron powder for laser powder bed fusion
Taehyeob Im, Kopila Gurung, Sebastian Meyers, Antonio Cutolo, Huengseok Oh, Jai-Sung Lee, Brecht Van Hooreweder, Caroline Sunyong Lee
Journal of Materials Processing Technology.2022; 308: 117705. CrossRef
- A new strategy for metal additive manufacturing using an economical water-atomized iron powder for laser powder bed fusion
- [Korean]
- Synthesis and Characterization of Core-Shell Silica-Phosphor Nanoparticles
via Sol-Gel Process - Weon Ho Shin, Seyun Kim, Hyung Mo Jeong
- J Korean Powder Metall Inst. 2018;25(1):12-18. Published online February 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.1.12
- 1,001 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
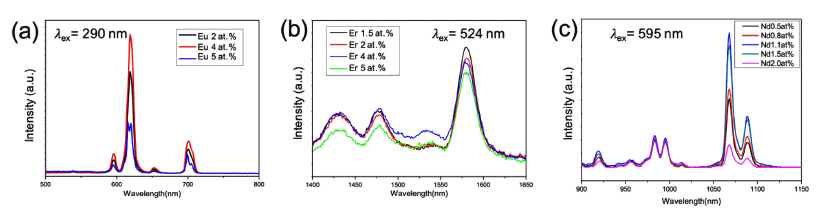
PDF Cost-effective functional phosphor nanoparticles are prepared by introducing low-cost SiO2 spheres to rareearth phosphor (YVO4:Eu3+, YVO4:Er3+, and YVO4:Nd3+) shells using a sol-gel synthetic method. These functional nanoparticles are characterized by X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, and general photoluminescence spectra. The SiO2 sphere occupying the interior of the conventional phosphor is advantageous in significantly reducing the cost of expensive rare-earth phosphor nanoparticles. The sol-gel process facilitates the core–shell structure formation; the rare-earth shell phosphor has strong interactions with chelating agents on the surfaces of SiO2 nanoparticles and thus forms layers of several nanometers in thickness. The photoluminescence wavelength is simply tuned by replacing the active materials of Eu3+, Er3+, and Nd3+. Moreover, the photoluminescent properties of the core–shell nanoparticles can be optimized by manipulating the specific contents of active materials in the phosphors. Our simple approach substitutes low-cost SiO2 for expensive rare-earth-based phosphor materials to realize cost-effective phosphor nanoparticles for various applications.
-
Citations
Citations to this article as recorded by- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
Woo Hyeong Sim, Seyun Kim, Weon Ho Shin, Hyung Mo Jeong
Korean Journal of Metals and Materials.2020; 58(2): 137. CrossRef
- Enhanced Energy-Transfer Properties in Core-Shell Photoluminescent Nanoparticles Using Mesoporous SiO2 Intermediate Layers
- [Korean]
- Preparation of Nanosized Gd2O3:Eu3+ Red Phosphor Coated on Mica Flake and Its Luminescent Property
- Se-Min Ban, Jeong Min Park, Kyeong Youl Jung, Byung-Ki Choi, Kwang-Jung Kang, Myung Chang Kang, Dae-Sung Kim
- J Korean Powder Metall Inst. 2017;24(6):457-463. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.457

- 970 View
- 4 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Nanosized Gd2O3:Eu3+ red phosphor is prepared using a template method from metal salt impregnated into a crystalline cellulose and is dispersed using a bead mill wet process. The driving force of the surface coating between Gd2O3:Eu3+ and mica is induced by the Coulomb force. The red phosphor nanosol is effectively coated on mica flakes by the electrostatic interaction between positively charged Gd2O3:Eu3+ and negatively charged mica above pH 6. To prepare Gd2O3:Eu3+-coated mica (Gd2O3:Eu/mica), the coating conditions are optimized, including the stirring temperature, pH, calcination temperature, and coating amount (wt%) of Gd2O3:Eu3+. In spite of the low luminescence of the Gd2O3:Eu/mica, the luminescent property is recovered after calcination above 600°C and is enhanced by increasing the Gd2O3:Eu3+ coating amount. The Gd2O3:Eu/mica is characterized using X-ray diffraction, field emission scanning electron microscopy, zeta potential measurements, and fluorescence spectrometer analysis.
-
Citations
Citations to this article as recorded by- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
Mahboob Ullah, Se-Min Ban, Dae-Sung Kim
Optical Materials.2019; 97: 109366. CrossRef
- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
- [Korean]
- Current Technology Trends Analysis on the Recovery of Rare Earth Elements from Fluorescent Substance in the Cold Cathode Fluorescent Lamps of Waste Flat Panel Displays
- Leeseung Kang, Dongyoon Shin, Jieun Lee, Joong Woo Ahn, Hyun-Seon Hong
- J Korean Powder Metall Inst. 2015;22(1):27-31. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.27

- 750 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Flat panel display devices are mainly used as information display devices in the 21st century. The worldwide waste flat panel displays are expected at 2-3 million units but most of them are land-filled for want of a proper recycling technology More specifically, rare earth metals of La and Eu are used as fluorescent materials of Cold Cathode Flourscent Lamp(CCFL)s in the waste flat panel displays and they are critically vulnerable and irreplaceable strategic mineral resources. At present, most of the waste CCFLs are disposed of by land-filling and incineration and proper recovery of 80-plus tons per annum of the rare earth fluorescent materials will significantly contribute to steady supply of them. A dearth of Korean domestic research results on recovery and recycling of rare earth elements in the CCFLs prompts to initiate this status report on overseas research trends and noteworthy research results in related fields.
TOP
 KPMI
KPMI


 First
First Prev
Prev


