Search
- Page Path
- HOME > Search
- [Korean]
- A Study on the Microstructures and Ionic Conductivity of Li1.3Al0.3Ti1.7(PO4)3 with Different Synthesis Routes
- Seul Ki Choi, Jeawon Choi, MinHo Yang
- J Powder Mater. 2023;30(2):107-115. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.107
- 2,074 View
- 47 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
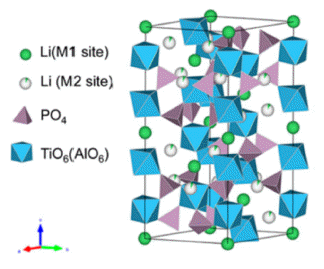
PDF Li1.3Al0.3Ti1.7(PO4)3(LATP) is considered a promising material for all-solid-state lithium batteries owing to its high moisture stability, wide potential window (~6 V), and relatively high ion conductivity (10-3–10-4 S/cm). Solid electrolytes based on LATP are manufactured via sintering, using LATP powder as the starting material. The properties of the starting materials depend on the synthesis conditions, which affect the microstructure and ionic conductivity of the solid electrolytes. In this study, we synthesize the LATP powder using sol-gel and co-precipitation methods and characterize the physical properties of powder, such as size, shape, and crystallinity. In addition, we have prepared a disc-shaped LATP solid electrolyte using LATP powder as the starting material. In addition, X-ray diffraction, scanning electron microscopy, and electrochemical impedance spectroscopic measurements are conducted to analyze the grain size, microstructures, and ion conduction properties. These results indicate that the synthesis conditions of the powder are a crucial factor in creating microstructures and affecting the conduction properties of lithium ions in solid electrolytes.
-
Citations
Citations to this article as recorded by- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
Gwanhee Song, Bojoong Kim, Inkook Hwang, Jiwon Kim, Jinmo Kim, Chang-Bun Yoon
Materials.2025; 18(3): 609. CrossRef
- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
- [Korean]
- Cobalt Recovery by Oxalic Acid and Hydroxide Precipitation from Waste Cemented Carbide Scrap Cobalt Leaching Solution
- Jaesung Lee, Mingoo Kim, Seulgi Kim, Dongju Lee
- J Korean Powder Metall Inst. 2021;28(6):497-501. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.497
- 1,272 View
- 17 Download
-
 Abstract
Abstract
 PDF
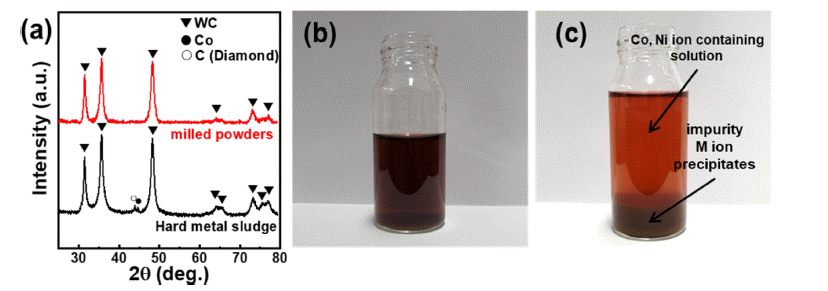
PDF Cobalt (Co) is mainly used to prepare cathode materials for lithium-ion batteries (LIBs) and binder metals for WC-Co hard metals. Developing an effective method for recovering Co from WC-Co waste sludge is of immense significance. In this study, Co is extracted from waste cemented carbide soft scrap via mechanochemical milling. The leaching ratio of Co reaches approximately 93%, and the leached solution, from which impurities except nickel are removed by pH titration, exhibits a purity of approximately 97%. The titrated aqueous Co salts are precipitated using oxalic acid and hydroxide precipitation, and the effects of the precipitating agent (oxalic acid and hydroxide) on the cobalt microstructure are investigated. It is confirmed that the type of Co compound and the crystal growth direction change according to the precipitation method, both of which affect the microstructure of the cobalt powders. This novel mechanochemical process is of significant importance for the recovery of Co from waste WC-Co hard metal. The recycled Co can be applied as a cemented carbide binder or a cathode material for lithium secondary batteries.
- [Korean]
- Study on preparation and photocatalytic properties of F-containing TiO2 nanopowders using wet-process from Ammonium Hexafluorotitanate
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Hyeon-Mo Kim, Kyung-Soo Park
- J Korean Powder Metall Inst. 2018;25(3):226-331. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.226

- 510 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF F-containing TiO2 nanopowders are synthesized using simple wet processes (precipitation-based and hydrothermal) from ammonium hexafluorotitanate (AHFT, (NH4)2TiF6) as a precursor to apply as a photocatalyst for the degradation of rhodamine B (RhB). The surface properties of the prepared samples are evaluated using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). The results confirm that the synthesized anatase TiO2 has sphere-like shapes, with numerous small nanoparticles containing fluorine on the surface. The photocatalytic activity of F-containing TiO2 compared with F-free TiO2 is characterized by measuring the degradation of RhB using a xenon lamp. The photocatalytic degradation of F-containing TiO2 exhibits improved photocatalytic activity, based on the positive effects of adsorbed F ions on the surface.
- [Korean]
- Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
- Woonghee Choi, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(4):287-296. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.287
- 1,814 View
- 33 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
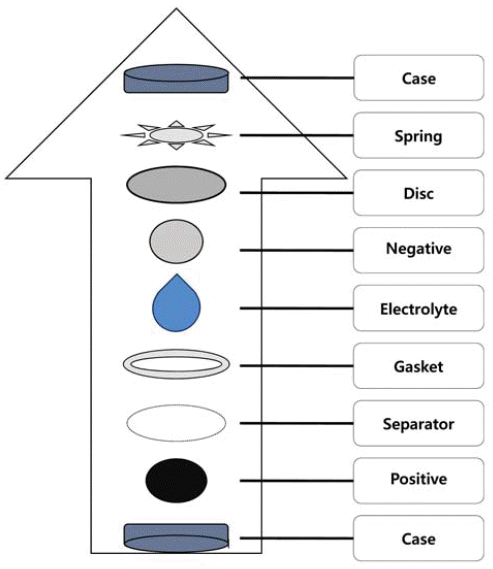
PDF Ni1/3Co1/3Mn1/3(OH)2 powders have been synthesized in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH using NH4OH as a chelating agent. The co-precipitation temperature is varied in the range of 30-80°C. Calcination of the prepared precursors with Li2CO3 for 8 h at 1000°C in air results in Li Ni1/3Co1/3Mn1/3O2 powders. Two kinds of obtained powders have been characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analyzer, and tap density measurements. The co-precipitation temperature does not differentiate the XRD patterns of precursors as well as their final powders. Precursor powders are spherical and dense, consisting of numerous acicular or flaky primary particles. The precursors obtained at 70 and 80°C possess bigger primary particles having more irregular shapes than those at lower temperatures. This is related to the lower tap density measured for the former. The final powders show a similar tendency in terms of primary particle shape and tap density. Electrochemical characterization shows that the initial charge/discharge capacities and cycle life of final powders from the precursors obtained at 70 and 80°C are inferior to those at 50°C. It is concluded that the optimum co-precipitation temperature is around 50°C.
-
Citations
Citations to this article as recorded by- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
Seon Hwa Lee, Ki Young Kwon, Byeong Kil Choi, Hyun Deog Yoo
Journal of Electroanalytical Chemistry.2022; 924: 116828. CrossRef
- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
- [Korean]
- Characteristics of Ni1/3Co1/3Mn1/3(OH)2 Powders Prepared by Co-Precipitation in Air and Nitrogen Atmospheres
- Woonghee Choi, Se-Ryen Park, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(2):136-142. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.136
- 2,649 View
- 53 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
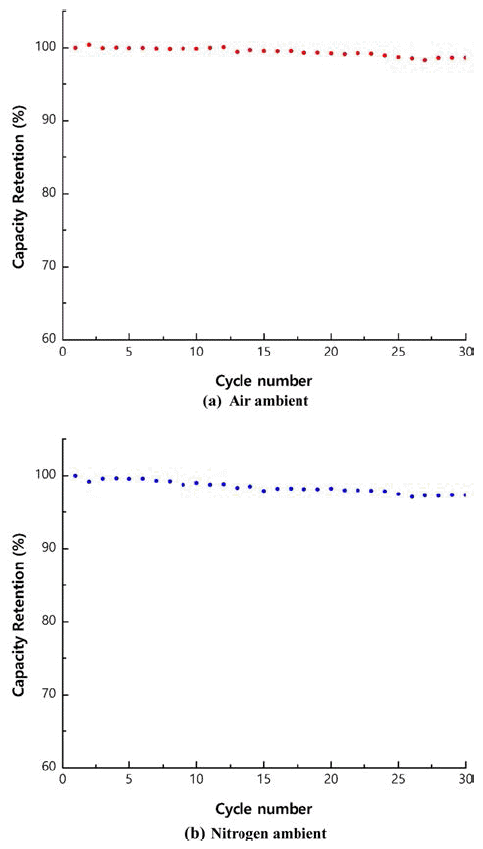
PDF As precursors of cathode materials for lithium ion batteries, Ni1/3Co1/3Mn1/3(OH)2 powders are prepared in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH in the presence of NH4OH in air or nitrogen ambient. Calcination of the precursors with Li2CO3 for 8 h at 1,000°C in air produces dense spherical cathode materials. The precursors and final powders are characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analysis, tap density measurement, and thermal gravimetric analysis. The precursor powders obtained in air or nitrogen ambient show XRD patterns identified as Ni1/3Co1/3Mn1/3(OH)2. Regardless of the atmosphere, the final powders exhibit the XRD patterns of LiNi1/3Co1/3Mn1/3O2 (NCM). The precursor powders obtained in air have larger particle size and lower tap density than those obtained in nitrogen ambient. NCM powders show similar tendencies in terms of particle size and tap density. Electrochemical characterization is performed after fabricating a coin cell using NCM as the cathode and Li metal as the anode. The NCM powders from the precursors obtained in air and those from the precursors obtained in nitrogen have similar initial charge/discharge capacities and cycle life. In conclusion, the powders co-precipitated in air can be utilized as precursor materials, replacing those synthesized in the presence of nitrogen injection, which is the usual industrial practice.
-
Citations
Citations to this article as recorded by- Thermodynamic modeling and parameters optimization for Ni0.8Co0.1Mn 0.1(OH)2 synthesis via transitional metal coprecipitation
Zanlang Tang, Chen Liu, Xincun Tang, Haonan Liu, Biao Qin
Materials Science and Engineering: B.2026; 326: 119205. CrossRef - Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
Minjeong Kim, Soonhyun Hong, Heongkwon Jeon, Jahun Koo, Heesang Lee, Gyuseok Choi, Chunjoong Kim
Korean Journal of Materials Research.2022; 32(4): 216. CrossRef - Spherical agglomeration of nickel-manganese-cobalt hydroxide in turbulent Batchelor vortex flow
Xiaotong Sun, Jinsoo Kim, Woo-Sik Kim
Chemical Engineering Journal.2021; 421: 129924. CrossRef - Design strategies for development of nickel-rich ternary lithium-ion battery
Kyu Hwan Choi, Xuyan Liu, Xiaohong Ding, Qiang Li
Ionics.2020; 26(3): 1063. CrossRef - Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 49. CrossRef - Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
Woonghee Choi, Chan Hyoung Kang
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 287. CrossRef
- Thermodynamic modeling and parameters optimization for Ni0.8Co0.1Mn 0.1(OH)2 synthesis via transitional metal coprecipitation
TOP
 KPMI
KPMI


 First
First Prev
Prev


