Search
- Page Path
- HOME > Search
- [Korean]
- Fabrication and Alloying Behavior of Ultra-Lightweight AlTiCrVMg High-Entropy Alloy via Al-Mg Mutual Solubility and Sintering Control
- Eunhyo Song, Hansung Lee, Byungmin Ahn
- J Powder Mater. 2025;32(3):254-261. Published online June 12, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00059

- 652 View
- 23 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - High-entropy alloys (HEAs) incorporating low-melting-point elements (Mg and Al) and high-melting-point elements (Ti, Cr, and V) were fabricated via mechanical alloying and spark plasma sintering. Sintering temperatures were varied to investigate phase behavior and microstructural evolution. X-ray diffraction was used to identify phase structures, scanning electron microscopy to analyze microstructures, X-ray fluorescence to determine elemental composition, and a gas pycnometer to measure density. Micro-Vickers hardness testing was conducted to evaluate mechanical properties. Mechanical-alloyed HEAs exhibited a body-centered cubic (BCC) phase and lamellar structures with element-enriched regions. Sintering introduced additional BCC and Laves phases, while higher temperatures promoted Mg liquid-phase sintering, increasing density and hardness. This study highlights the effects of sintering on HEAs containing elements with differing melting points to optimize their properties.
-
Citations
Citations to this article as recorded by- Effect of annealing temperature on thermal expansion and cryogenic mechanical properties of low-thermal-expansion Co22.2Cr6.2Fe48.8Ni17.8Cu5.0 medium-entropy alloy
Wooyoung Lee, Munsu Choi, Sungwook Kim, Dae-Kyeom Kim, Myungsuk Song, Taek-Soo Kim, Jungwan Lee, Hyoung Seop Kim, Hyunjoo Choi, Soo-Hyun Joo
Materials Science and Engineering: A.2026; 954: 149811. CrossRef
- Effect of annealing temperature on thermal expansion and cryogenic mechanical properties of low-thermal-expansion Co22.2Cr6.2Fe48.8Ni17.8Cu5.0 medium-entropy alloy
- [Korean]
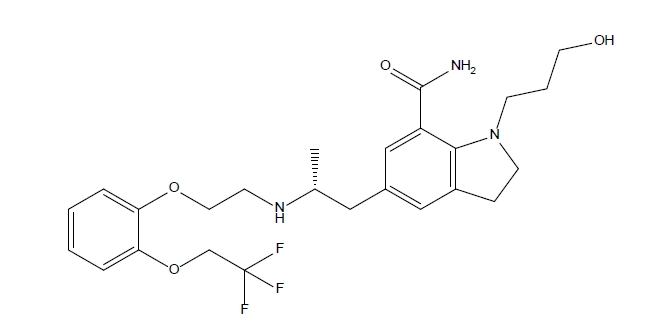
- Comparison and Characterization of Silodosin-loaded Solid Dispersions Prepared by Various Solid Dispersion Preparation Methods
- Su Man Lee, Da Young Song, Kyeong Soo Kim
- J Powder Mater. 2024;31(3):263-271. Published online June 27, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00143
- 1,581 View
- 37 Download
-
 Abstract
Abstract
 PDF
PDF - This study focused on improving the solubility of silodosin, a drug poorly soluble in water, by utilizing solid dispersions. Three types of dispersions were examined and compared against the drug powder: surface-attached (SA), solvent-wetted (SW), and solvent-evaporated (SE). Polyvinyl alcohol (PVA) was identified as the most effective polymer in enhancing solubility. These dispersions were prepared using spray-drying techniques with silodosin and PVA as the polymer, employing solvents such as water, ethanol, and a water-acetone mix. The physicochemical properties and solubility of the dispersions were evaluated. The surface-attached dispersions featured the polymer on a crystalline drug surface, the solvent-wetted dispersions had the amorphous drug on the polymer, and the solvent-evaporated dispersions produced nearly round particles with both components amorphous. Testing revealed that the order of improved solubility was: solvent-evaporated, solvent-wetted, and surface-attached. The results demonstrated that the preparation method of the solid dispersions significantly impacted their physicochemical properties and solubility enhancement.
- [Korean]
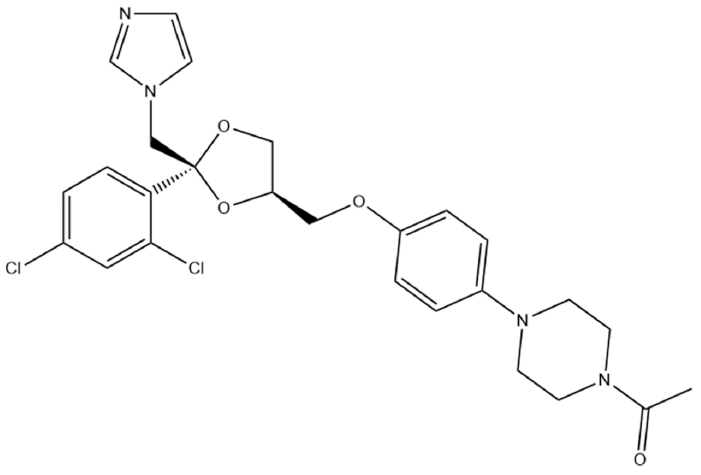
- Preparation and Evaluation of Ketoconazole-loaded Solid-SNEDDS (Self-Nanoemulsifying Drug Delivery System) using Various Solidification Carriers
- Da Young Song, Kyeong Soo Kim
- J Powder Mater. 2023;30(6):493-501. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.493
- 982 View
- 10 Download
-
 Abstract
Abstract
 PDF
PDF This study aimed to develop a solid self-nanoemulsifying drug delivery system (solid-SNEDDS) to enhance the formulation of ketoconazole (KTZ), a BCS Class II drug with poor solubility. Ketoconazole, which is insoluble above pH 3, requires solubilization for effective delivery. This SNEDDS comprises oil, surfactant, and co-surfactant, which spontaneously emulsify in the gastrointestinal tract environment to form nanoemulsions with droplet sizes less than 100 nm. The optimal SNE-vehicle composition of oleic acid, TPGS, and PEG 400 at a 10:80:10 weight ratio was determined based on the smallest droplet size achieved. This composition was used to prepare liquid SNEDDS containing ketoconazole. The droplet size and polydispersity index (PDI) of the resulting liquid SNEDDS were analyzed. Subsequently, solid-SNEDDS was fabricated using a spray-drying method with solidifying carriers such as silicon dioxide, crospovidone, and magnesium alumetasilicate. The physicochemical properties of the solid-SNEDDS were characterized by scanning electron microscopy and powder X-ray diffraction, and its solubility, droplet size, and PDI were evaluated. In particular, the solid-SNEDDS containing ketoconazole and crospovidone in a 2:1 weight ratio exhibited significantly enhanced solubility, highlighting its potential for improved medication adherence and dissolution rates.
- [Korean]
- Fabrication and Evaluation of Colloidal Silica Containing Powders for Solid Self-emulsifying Drug Delivery System of Poorly Water Soluble Rivaroxaban
- Sung Giu Jin
- J Powder Mater. 2023;30(4):305-309. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.305

- 788 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF This study aims to prepare a colloidal silica-containing powder to enhance the solubility and dissolution rate of rivaroxaban using a self-nanoemulsifying drug delivery system (SNEDDS). We investigate the impact of colloidal silica on a nanoemulsion system for preparing powdered SNEDDS. The liquid SNEDDS comprises 30/20/50 (w/w/w) Peceol/ Cremophor RH40/Tween 80, which results in the formation of the smallest droplets. Three powdered SNEDDS formulations are prepared by suspending the liquid SNEDDS formulation using colloidal silica and spray drying. The powdered SNEDDS prepared with liquid SNEDDS and colloidal silica at a ratio of 1/0.5 (w/w) exhibits the highest water solubility (0.94 ± 0.62 vs. 26.70 ± 1.81 μg/mL) and dissolution rate (38.4 ± 3.6 vs. 85.5 ± 3.4%, 45 min) when compared to the drug alone. Morphologically, the liquid SNEDDS is adsorbed onto colloidal silica and forms smaller particles. In conclusion, an SNEDDS containing rivaroxaban, prepared using colloidal silica, facilitates the creation of a nanoemulsion and enhances the water solubility of rivaroxaban. Accordingly, this technology holds significant potential for commercialization.
- [Korean]
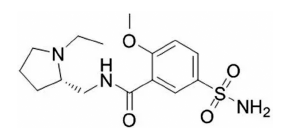
- Fabrication and Evaluation of Levosulpiride-loaded Amorphous Spray-dried Microparticle for Improved Solubility
- Sung Giu Jin
- J Powder Mater. 2023;30(1):47-52. Published online February 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.1.47
- 835 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF The purpose of this study is to develop and evaluate amorphous spray-dried microparticles (SDM) containing levosulpiride to increase its solubility. SDM are prepared via solvent evaporation using polyvinylpyrrolidone (PVP) as the water-soluble polymer and Cremophor RH40 as the surfactant. The SDM is prepared by varying the amounts of PVP and Cremophor RH40, and its physicochemical properties, solubility, and dissolution are confirmed. All levosulpiride-loaded SDMs converted the crystalline drug into an amorphous form, significantly improving drug solubility and dissolution compared with the drug alone. SDM consisting of drug/PVP/Cremophor RH40 in a weight ratio of 5:10:3, with increased solubility (720 ± 36 vs. 1822 ± 51 μg/mL) and dissolution rate (10.3 ± 2.2 vs. 92.6 ± 6.0%) compared with drug alone, shows potential as a commercial drug for improved oral bioavailability of levosulpiride.
TOP
 KPMI
KPMI


 First
First Prev
Prev


