Search
- Page Path
- HOME > Search
- [Korean]
- Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
- Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2018;25(5):395-401. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.395
- 2,118 View
- 46 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
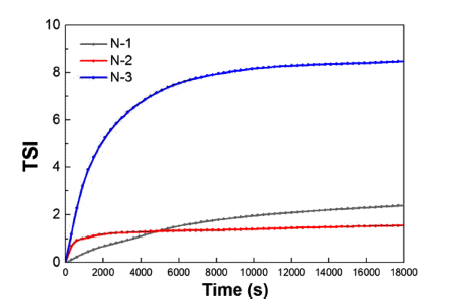
PDF The improvement of dispersion stability for the primary polishing slurry in a CMP process is achieved to prevent defects produced by agglomeration of the slurry. The dispersion properties are analyzed according to the physical characteristics of each silica sol sample. Further, the difference in the dispersion stability is confirmed as the surfactant content. The dispersibility results measured by Zeta potential suggest that the dispersion properties depend on the content and size of the abrasive in the primary polishing slurry. Moreover, the optimum ratio for high dispersion stability is confirmed as the addition content of the surfactant. Based on the aforementioned results, the long-term stability of each slurry is analyzed. Turbiscan analysis demonstrates that the agglomeration occurs depending on the increasing amount of surfactant. As a result, we demonstrate that the increased particle size and the decreased content of silica improve the dispersion stability and long-term stability.
-
Citations
Citations to this article as recorded by- Surface Defect Properties of Prime, Test-Grade Silicon Wafers
Seung-Hwan Oh, Hyeonmin Yim, Donghee Lee, Dong Hyeok Seo, Won Jin Kim, Ryun Na Kim, Woo-Byoung Kim
Korean Journal of Materials Research.2022; 32(9): 396. CrossRef
- Surface Defect Properties of Prime, Test-Grade Silicon Wafers
- [Korean]
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
- J Korean Powder Metall Inst. 2018;25(3):257-262. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.257
- 1,136 View
- 9 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
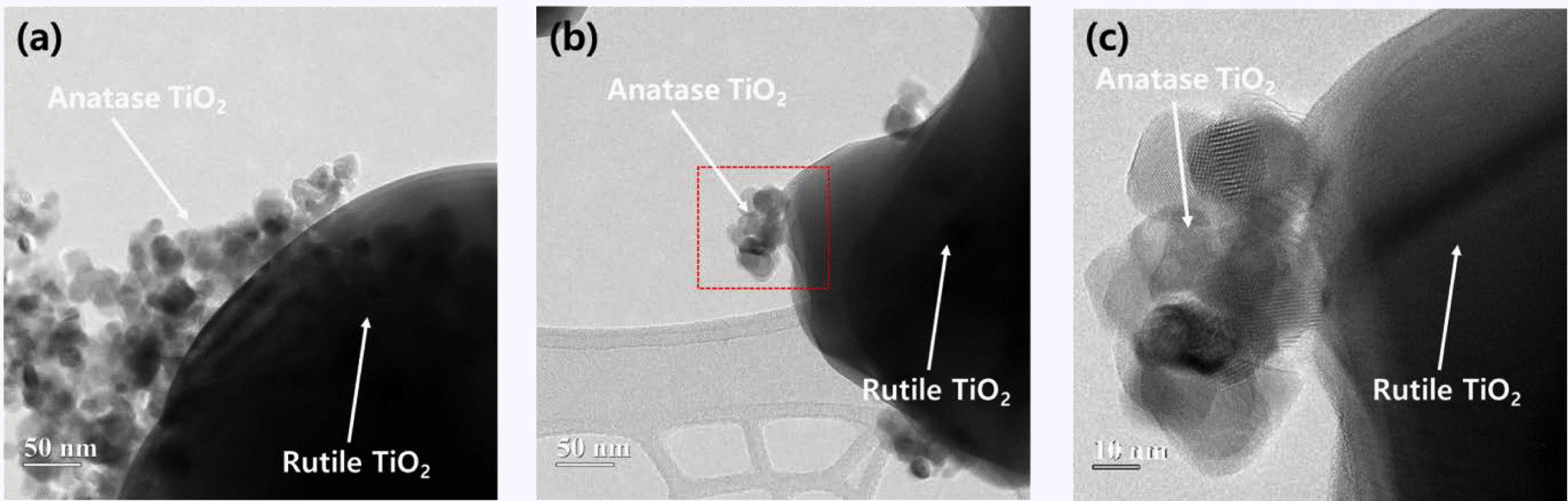
PDF The coupling of two semiconducting materials is an efficient method to improve photocatalytic activity via the suppression of recombination of electron-hole pairs. In particular, the coupling between two different phases of TiO2, i.e., anatase and rutile, is particularly attractive for photocatalytic activity improvement of rutile TiO2 because these coupled TiO2 powders can retain the benefits of TiO2, one of the best photocatalysts. In this study, anatase TiO2 nanoparticles are synthesized and coupled on the surface of rutile TiO2 powders using a microemulsion method and heat treatment. Triton X-100, as a surfactant, is used to suppress the aggregation of anatase TiO2 nanoparticles and disperse anatase TiO2 nanoparticles uniformly on the surface of rutile TiO2 powders. Rutile TiO2 powders coupled with anatase TiO2 nanoparticles are successfully prepared. Additionally, we compare the photocatalytic activity of these rutile-anatase coupled TiO2 powders under ultraviolet (UV) light and demonstrate that the reason for the improvement of photocatalytic activity is microstructural.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 395. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
- [Korean]
- The Preparation and Growth Mechanism of the Recovered Bi2Te3 Particles with Respect to Surfactants
- Hyeongsub So, Eunpil Song, Yong-Ho Choa, Kun-Jae Lee
- J Korean Powder Metall Inst. 2017;24(2):141-146. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.141

- 1,186 View
- 9 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Bi2Te3 powders are recovered by wet chemical reduction for waste n-type thermoelectric chips, and the recovered particles with different morphologies are prepared using various surfactants such as cetyltrimethylammonium bromide (CTAB), sodium dodecylbenzenesulfonate (SDBS), and ethylenediaminetetraacetic acid (EDTA). When citric acid is added as the surfactant, the shape of the aggregated particles shows no distinctive features. On the other hand, rod-shaped particles are formed in the sample with CTAB, and sheet-like particles are synthesized with the addition of SDBS. Further, particles with a tripod shape are observed when EDTA is added as the surfactant. The growth mechanism of the particle shapes depending on the surfactant is investigated, with a focus on the nucleation and growth phenomena. These results help to elucidate the intrinsic formation mechanism of the rod, plate, and tripod structures of the Bi2Te3 recovered by the wet reduction process.
-
Citations
Citations to this article as recorded by- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
Haein Shin, Kun-Jae Lee
Journal of Powder Materials.2024; 31(5): 374. CrossRef
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- [Korean]
- Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
- Il-Kyu Park
- J Korean Powder Metall Inst. 2016;23(4):270-275. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.270
- 872 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
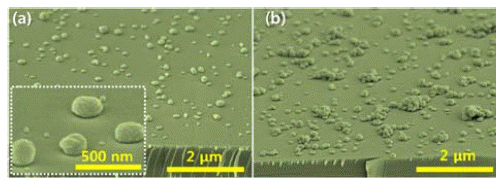
PDF We report on an all-solution-processed hydrothermal method to control the morphology of ZnO nanostructures on Si substrates from three-dimensional hemispherical structures to two-dimensional thin film layers, by controlling the seed layer and the molar contents of surfactants during their primary growth. The size and the density of the seed layer, which is composed of ZnO nanodots, change with variation in the solute concentration. The ZnO nanodots act as heterogeneous nucleation sites for the main ZnO nanostructures. When the seed layer concentration is increased, the ZnO nanostructures change from a hemispherical shape to a thin film structure, formed by densely packed ZnO hemispheres. In addition, the morphology of the ZnO layer is systematically controlled by using trisodium citrate, which acts as a surfactant to enhance the lateral growth of ZnO crystals rather than a preferential one-dimensional growth along the c-direction. X-ray diffraction and energy dispersive X-ray spectroscopy results reveal that the ZnO structure is wurtzite and did not incorporate any impurities from the surfactants used in this study.
-
Citations
Citations to this article as recorded by- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
Leeseung Kang, HyeLan An, Ji Young Park, Myung Hwan Hong, Sahn Nahm, Chan Gi Lee
Applied Surface Science.2019; 475: 969. CrossRef
- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
TOP
 KPMI
KPMI


 First
First Prev
Prev


