Search
- Page Path
- HOME > Search
- [Korean]
- Fabrication and Characterization of Hexagonal Tungsten Oxide Nanopowders for High Performance Gas Sensing Application
- Jinsoo Park
- J Korean Powder Metall Inst. 2019;26(1):28-33. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.28
- 810 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF The gas sensor is essential to monitoring dangerous gases in our environment. Metal oxide (MO) gas sensors are primarily utilized for flammable, toxic and organic gases and O3 because of their high sensitivity, high response and high stability. Tungsten oxides (WO3) have versatile applications, particularly for gas sensor applications because of the wide bandgap and stability of WO3. Nanosize WO3 are synthesized using the hydrothermal method. Asprepared WO3 nanopowders are in the form of nanorods and nanorulers. The crystal structure is hexagonal tungsten bronze (MxWO3, x =< 0.33), characterized as a tunnel structure that accommodates alkali ions and the phase stabilizer. A gas detection test reveals that WO3 can detect acetone, butanol, ethanol, and gasoline. This is the first study to report this capability of WO3.
- [Korean]
- Photocatalysis of TiO2/WO3 Composites Synthesized by Ball Milling
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2018;25(4):316-321. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.316
- 759 View
- 3 Download
-
 Abstract
Abstract
 PDF
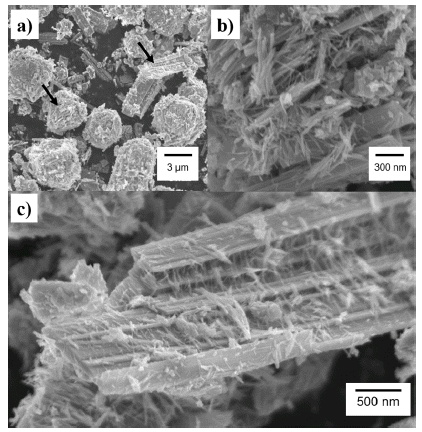
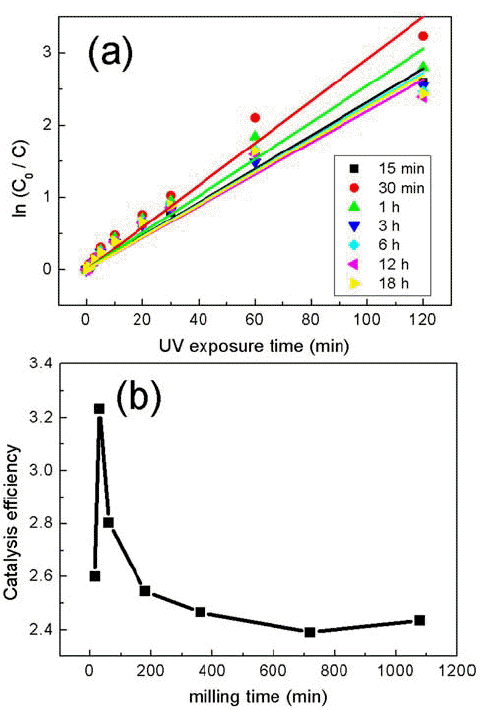
PDF Composites of P25 TiO2 and hexagonal WO3 nanorods are synthesized through ball-milling in order to study photocatalytic properties. Various composites of TiO2/WO3 are prepared by controlling the weight percentages (wt%) of WO3, in the range of 1–30 wt%, and milling time to investigate the effects of the composition ratio on the photocatalytic properties. Scanning electron microscopy, x-ray diffraction, and transmission electron microscopy are performed to characterize the structure, shape and size of the synthesized composites of TiO2/WO3. Methylene blue is used as a test dye to analyze the photocatalytic properties of the synthesized composite material. The photocatalytic activity shows that the decomposition efficiency of the dye due to the photocatalytic effect is the highest in the TiO2/WO3 (3 wt%) composite, and the catalytic efficiency decreases sharply when the amount of WO3 is further increased. As the amount of WO3 added increases, dye-removal by adsorption occurs during centrifugation, instead of the decomposition of dyes by photocatalysts. Finally, TiO2/WO3 (3 wt%) composites are synthesized with various milling times. Experimental results show that the milling time has the best catalytic efficiency at 30 min, after which it gradually decreases. There is no significant change after 1 hour.
- [Korean]
- Photocatalytic and Adsorption Properties of WO3 Nanorods Prepared by Hydrothermal Synthesis
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2017;24(6):483-488. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.483
- 1,108 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
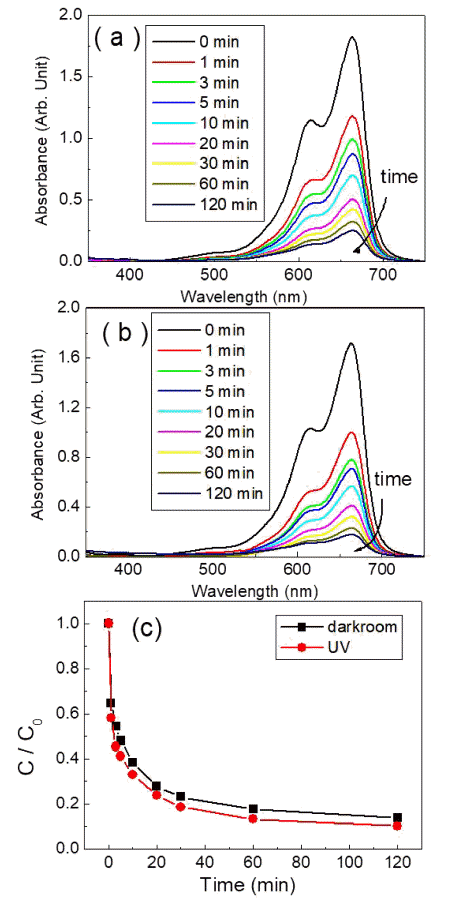
PDF Transition-metal oxide semiconductors have various band gaps. Therefore, many studies have been conducted in various application fields. Among these, methods for the adsorption of organic dyes and utilization of photocatalytic properties have been developed using various metal oxides. In this study, the adsorption and photocatalytic effects of WO3 nanomaterials prepared by hydrothermal synthesis are investigated, with citric acid added in the hydrothermal process as a structure-directing agent. The nanostructures of WO3 are studied using transmission electron microscopy and scanning electron microscopy images. The crystal structure is investigated using X-ray diffraction patterns, and the changes in the dye concentrations adsorbed on WO3 nanorods are measured with a UV-visible absorption spectrophotometer based on Beer-Lambert’s law. The methylene blue (MB) dye solution is subjected to acid or base conditions to monitor the change in the maximum adsorption amount in relation to the pH. The maximum adsorption capacity is observed at pH 3. In addition to the dye adsorption, UV irradiation is carried out to investigate the decomposition of the MB dye as a result of photocatalytic effects. Significant photocatalytic properties are observed and compared with the adsorption effects for dye removal.
-
Citations
Citations to this article as recorded by- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
Kwang-Mo Kang, Ji-Hye Jeong, Ga-In Lee, Jae-Min Im, Hyun-Jeong Cheon, Deok-Hyeon Kim, Yoon-Chae Nah
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 40. CrossRef - Photocatalysis of TiO<sub>2</sub>/WO<sub>3</sub> Composites Synthesized by Ball Milling
Su-Yeol Yu, Chunghee Nam
Journal of Korean Powder Metallurgy Institute.2018; 25(4): 316. CrossRef
- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
TOP
 KPMI
KPMI


 First
First Prev
Prev


