Search
- Page Path
- HOME > Search
- [Korean]
- Aqueous Synthesis and Luminescent Characteristics of Cu:ZnSe Quantum Dots by Internal Doping Method
- Geum Ji Back, Hyun Seon Hong
- J Powder Mater. 2022;29(5):370-375. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.370
- 830 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
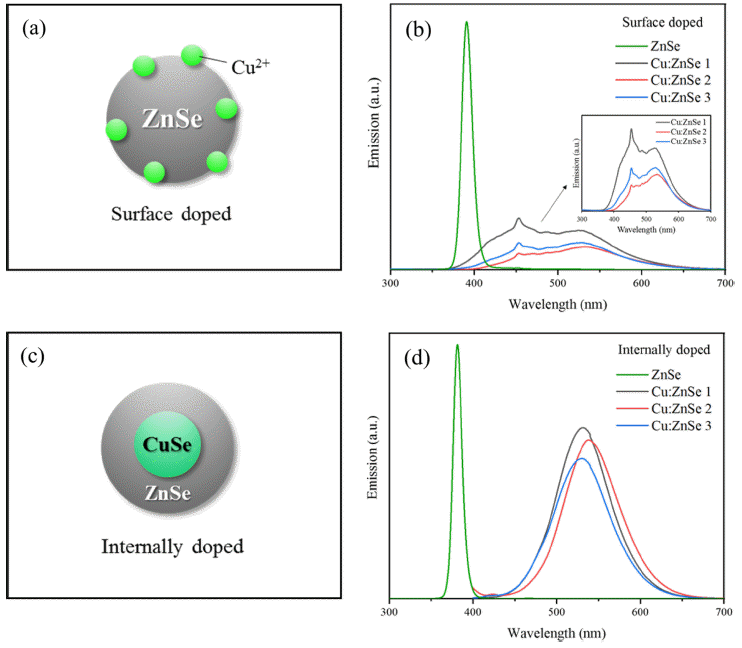
PDF Cu-doped ZnSe quantum dots were successfully synthesized in an aqueous solution using an internal doping method. The effects of ligand type, CuSe synthesis temperature, and heating time on Cu-doped ZnSe synthesis were systematically investigated. Of MPA, GSH, TGA, and NAC used as ligands, MPA was the optimal ligand as determined by PL spectrum analysis. In addition, the emission wavelength was found to depend on the synthesis temperature of the internal doping core of CuSe. As the temperature increased, the doping of Cu2+ was enhanced, and the emission wavelength band was redshifted; accordingly, the emission peaks moved from blue to green (up to 550 nm). Thus, the synthesis of Cu:ZnSe using internal doping in aqueous solutions is a potential method for ecomanufacturing of colortuned ZnSe quantum dots for display applications.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
- [Korean]
- Effects of Synthesis Conditions on Luminescence Characteristics of Glutathione Capped ZnSe Nano particles
- Geum Ji Back, Ha Yeon Song, Min Seo Lee, Hyun Seon Hong
- J Korean Powder Metall Inst. 2021;28(1):44-50. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.44
- 1,170 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
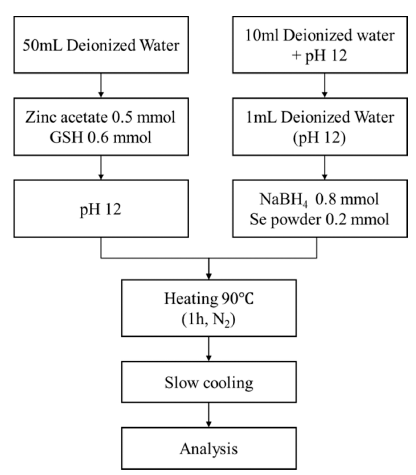
PDF Zinc selenide (ZnSe) nanoparticles were synthesized in aqueous solution using glutathione (GSH) as a ligand. The influence of the ligand content, reaction temperature, and hydroxyl ion concentration (pH) on the fabrication of the ZnSe particles was investigated. The optical properties of the synthesized ZnSe particles were characterized using various analytical techniques. The nanoparticles absorbed UV-vis light in the range of 350-400 nm, which is shorter than the absorption wavelength of bulk ZnSe particles (460 nm). The lowest ligand concentration for achieving good light absorption and emission properties was 0.6 mmol. The reaction temperature had an impact on the emission properties; photoluminescence spectroscopic analysis showed that the photo-discharge characteristics were greatly enhanced at high temperatures. These discharge characteristics were also affected by the hydroxyl ion concentration in solution; at pH 13, sound emission characteristics were observed, even at a low temperature of 25°C. The manufactured nanoparticles showed excellent light absorption and emission properties, suggesting the possibility of fabricating ZnSe QDs in aqueous solutions at low temperatures.
-
Citations
Citations to this article as recorded by- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
Geum Ji Back, Ha Yeon Song, Min Seo Lee, Jaesik Yoon, Hyun Seon Hong
Journal of Crystal Growth.2024; 626: 127475. CrossRef - Effect of UV Irradiation on Optical Properties of Water-Based Synthetic Zinc Selenide Quantum Dots
Geum Ji Back, Yu Jin Kang, I Ju Kang, Jeong Hyeon Lim, Hyun Seon Hong
Korean Journal of Metals and Materials.2022; 60(2): 160. CrossRef
- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
- [Korean]
- Influence of Reducing Agents and Additives on the Synthesis of ZnSe Nanoparticles
- Geum Ji Back, Da Gyeong Lee, Min Seo Lee, Ha Yeon Song, Hyun Seon Hong
- J Korean Powder Metall Inst. 2020;27(3):233-240. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.233
- 606 View
- 1 Download
-
 Abstract
Abstract
 PDF
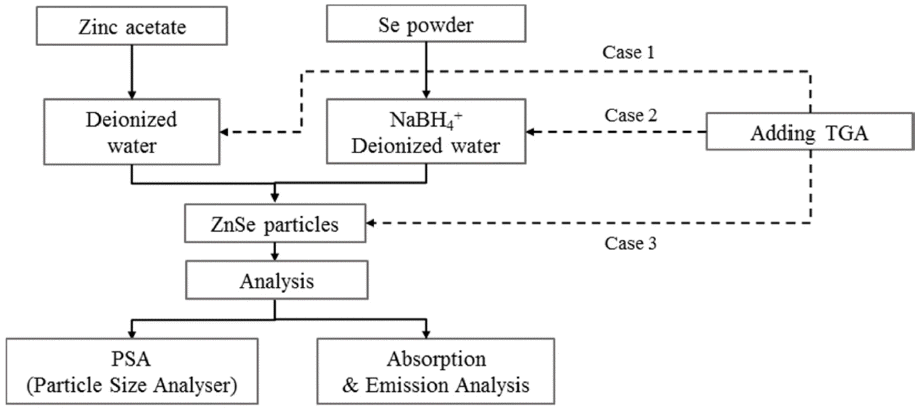
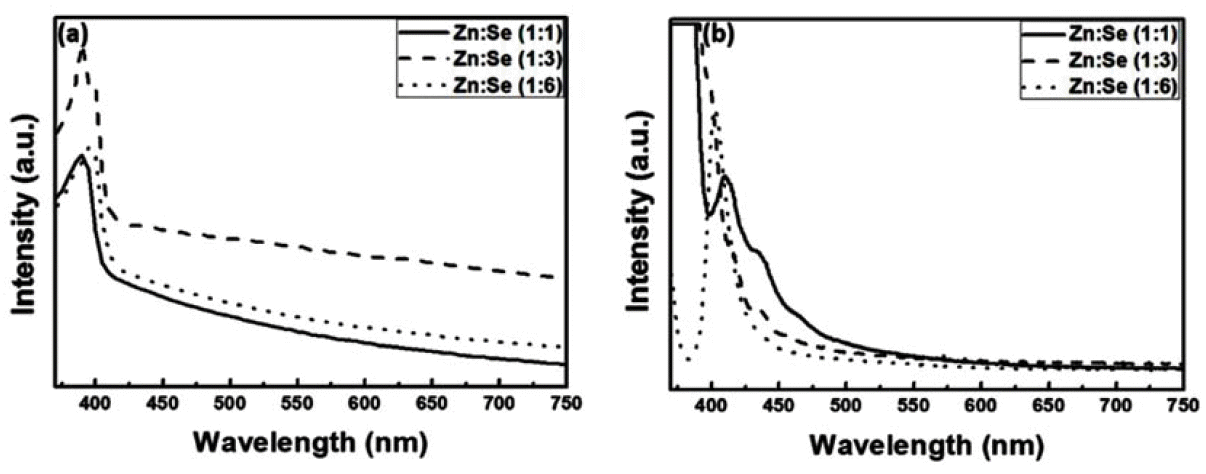
PDF Nano-sized ZnSe particles are successfully synthesized in an aqueous solution at room temperature using sodium borohydride (NaBH4) and thioglycolic acid (TGA) as the reducing agent and stabilizer, respectively. The effects of the mass ratio of the reducing agent to Se, stabilizer concentration, and stirring time on the synthesis of the ZnSe nanoparticles are evaluated. The light absorption/emission properties of the synthesized nanoparticles are characterized using ultraviolet-visible (UV-vis) spectroscopy, photoluminescence (PL) spectroscopy, and particle size analyzer (PSA) techniques. At least one mass ratio (NaBH4/Se) of the reducing agent should be added to produce ZnSe nanoparticles finer than 10 nm and to absorb UV–vis light shorter than the ZnSe bulk absorption wavelength of 460 nm. As the ratio of the reducing agent increases, the absorption wavelengths in the UV-vis curves are blue-shifted. Stirring in the atmosphere acts as a deterrent to the reduction reaction and formation of nanoparticles, but if not stirred in the atmosphere, the result is on par with synthesis in a nitrogen atmosphere. The stabilizer, TGA, has an impact on the Zn precursor synthesis. The fabricated nanoparticles exhibit excellent photo-absorption/discharge characteristics, suggesting that ZnSe nanoparticles can be alloyed without the need for organic solutions or high-temperature environments.
- [Korean]
- The Effect of Surface Defects on the Optical Properties of ZnSe:Eu Quantum Dots
- Da-Woon Jeong, Ji Young Park, Han Wook Seo, Kyoung-Mook Lim, Tae-Yeon Seong, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(5):348-352. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.348
- 1,289 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Quantum dots (QDs) are capable of controlling the typical emission and absorption wavelengths because of the bandgap widening effect of nanometer-sized particles. These phosphor particles have been used in optical devices, photovoltaic devices, advanced display devices, and several biomedical complexes. In this study, we synthesize ZnSe QDs with controlled surface defects by a heating-up method. The optical properties of the synthesized particles are analyzed using UV-visible and photoluminescence (PL) measurements. Calculations indicate nearly monodisperse particles with a size of about 5.1 nm at 260°C (full width at half maximum = 27.7 nm). Furthermore, the study results confirm that successful doping is achieved by adding Eu3+ preparing the growth phase of the ZnSe:Eu QDs when heating-up method. Further, we investigate the correlation between the surface defects and the luminescent properties of the QDs.
-
Citations
Citations to this article as recorded by- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
So-Yeong Joo, Hyun-Su Park, Do-yeon Kim, Bum-Sung Kim, Chan Gi Lee, Woo-Byoung Kim
AIP Advances.2018;[Epub] CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
TOP
 KPMI
KPMI


 First
First Prev
Prev


