Previous issues
- Page Path
- HOME > Browse Articles > Previous issues
- [Korean]
- A Study on Graphite Powder Compaction Behaviors Using the Discrete Element Method
- Jun Hyeok Jeong, Jinnil Choi
- J Korean Powder Metall Inst. 2021;28(1):1-6. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.1
- 997 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
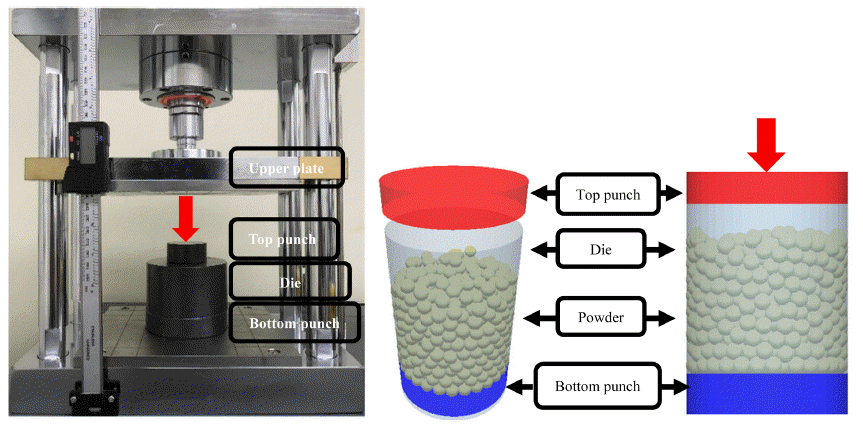
PDF Accurate and effective powder compaction analyses are performed for brittle materials such as graphite, utilized as a solid lubricant, by using the discrete element method (DEM). The reliability of the DEM analysis is confirmed by comparing the results of graphite powder compaction analyses using the DEM particle bonding contact model and particle non-bonding contact model with those from the powder compaction experiment under the same conditions. To improve the characteristics, the parameters influencing the compaction properties of the metal-graphite mixtures are explored. The compressibility increases as the size distribution of the graphite powder increases, where the shape of the graphite particles is uniform. The improved compaction characteristics of the metal-graphite (bonding model) mixtures are further verified by the stress transmission and compressive force distribution between the top and bottom punches. It is confirmed that the application of graphite (bonding model) powders resulted in improved stress transmission and compressive force distribution of 24% and 85%, respectively.
-
Citations
Citations to this article as recorded by- Effects of solid graphite lubricants for powder compaction
Jun Hyeok Jeong, Jinnil Choi
Powder Metallurgy.2021; 64(3): 241. CrossRef
- Effects of solid graphite lubricants for powder compaction
- [Korean]
- Effect of Change in Open Porosity as a Function of Uniaxial Molding Pressure on Density Improvement After Impregnation
- Sang-Min Lee, Sang-Hye Lee, Jae-Seung Roh
- J Korean Powder Metall Inst. 2021;28(1):7-12. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.7

- 1,620 View
- 23 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The change in the open porosity of bulk graphite as a function of the uniaxial molding pressure during manufacturing is studied using artificial graphite powder. Subsequently, the graphite is impregnated to determine the effect of the open porosity on the impregnation efficiency and to improve the density of the final bulk graphite. Bulk graphite is manufactured with different uniaxial molding pressures after mixing graphite powder, which is the by-product of processing the final graphite products and phenolic resin. The bulk density and open porosity are measured using the Archimedes method. The bulk density and open porosity of bulk graphite increase as the molding pressure increases. The open porosity of molded bulk graphite is 25.35% at 30 MPa and 29.84% at 300 MPa. It is confirmed that the impregnation efficiency increases when the impregnation process is performed on a specimen with large open porosity. In this study, the bulk density of bulk graphite molded at 300 MPa is 11.06% higher than that before impregnation, which is the highest reported increase. Therefore, it is expected that the higher the uniaxial pressure, the higher the density of bulk graphite.
-
Citations
Citations to this article as recorded by- Improving the packing and mechanical properties of graphite blocks by controlling filler particle-size distribution
Hye in Hwang, Ji Hong Kim, Ji Sun Im
Advanced Composite Materials.2024; 33(5): 762. CrossRef - Effect of Pressure and Holding Time during Compression Molding on Mechanical Properties and Microstructure of Coke-Pitch Carbon Blocks
Sun-Ung Gwon, Sang-Hye Lee, U-Sang Youn, Jae-Seung Roh
Applied Sciences.2024; 14(2): 772. CrossRef - Correlation between Pitch Impregnation Pressure and Pore Sizes of Graphite Block
Changkyu Kim, Woong Kwon, Moon Hee Lee, Jong Seok Woo, Euigyung Jeong
Materials.2022; 15(2): 561. CrossRef
- Improving the packing and mechanical properties of graphite blocks by controlling filler particle-size distribution
- [Korean]
- Mechanical Properties of Bulk Graphite using Artificial Graphite Scrap as a Function of Particle Size
- Sang Hye Lee, Sang Min Lee, Won Pyo Jang, Jae Seung Roh
- J Korean Powder Metall Inst. 2021;28(1):13-19. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.13
- 2,021 View
- 18 Download
- 7 Citations
-
 Abstract
Abstract
 PDF
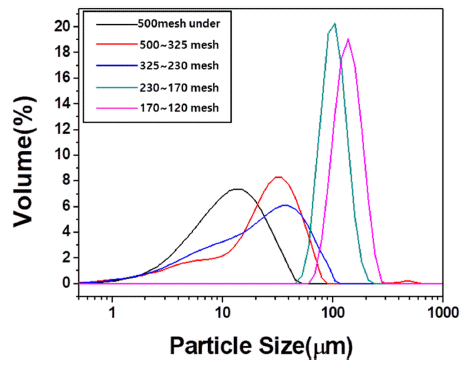
PDF Bulk graphite is manufactured using graphite scrap as the filler and phenolic resin as the binder. Graphite scrap, which is the by-product of processing the final graphite product, is pulverized and sieved by particle size. The relationship between the density and porosity is analyzed by measuring the mechanical properties of bulk graphite. The filler materials are sieved into mean particle sizes of 10.62, 23.38, 54.09, 84.29, and 126.64 μm. The bulk graphite density using the filler powder with a particle size of 54.09 μm is 1.38 g/cm3, which is the highest value in this study. The compressive strength tends to increase as the bulk graphite density increases. The highest compressive strength of 43.14 MPa is achieved with the 54.09 μm powder. The highest flexural strength of 23.08 MPa is achieved using the 10.62 μm powder, having the smallest average particle size. The compressive strength is affected by the density of bulk graphite, and the flexural strength is affected by the filler particle size of bulk graphite.
-
Citations
Citations to this article as recorded by- Effect of Microstructural Change under Pressure during Isostatic Pressing on Mechanical and Electrical Properties of Isotropic Carbon Blocks
Tae-Sub Byun, Sang-Hye Lee, Suk-Hwan Kim, Jae-Seung Roh
Materials.2024; 17(2): 387. CrossRef - Feasibility assessment of manufacturing carbonized blocks from rice husk charcoal
Young-Min Hwang, Jae-Seung Roh, Gibeop Nam
Biomass Conversion and Biorefinery.2024; 14(20): 26409. CrossRef - Improving the packing and mechanical properties of graphite blocks by controlling filler particle-size distribution
Hye in Hwang, Ji Hong Kim, Ji Sun Im
Advanced Composite Materials.2024; 33(5): 762. CrossRef - Effect of Impregnation and Graphitization on EDM Performance of Graphite Blocks Using Recycled Graphite Scrap
Sang-Hye Lee, Dong-Pyo Jeon, Hyun-Yong Lee, Dong-Gu Lee, Jae-Seung Roh
Processes.2023; 11(12): 3368. CrossRef - Ultrafine Graphite Scrap and Carbon Blocks Prepared by High-Solid-Loading Bead Milling and Conventional Ball Milling: A Comparative Assessment
Chonradee Amnatsin, Waroot Kanlayakan, Siraprapa Lhosupasirirat, Nattarut Verojpipath, Boonsueb Pragobjinda, Tanakorn Osotchan, Chakrit Sirisinha, Toemsak Srikhirin
ACS Omega.2023; 8(50): 47919. CrossRef - The Effect of the Heating Rate during Carbonization on the Porosity, Strength, and Electrical Resistivity of Graphite Blocks Using Phenolic Resin as a Binder
Sang-Hye Lee, Jae-Hyun Kim, Woo-Seok Kim, Jae-Seung Roh
Materials.2022; 15(9): 3259. CrossRef - Rheological Behaviour of Hard-Metal Carbide Powder Suspensions at High Shear Rates
B. Hausnerová, P. Sáha, J. Kubát, T. Kitano, J. Becker
Journal of Polymer Engineering.2000;[Epub] CrossRef
- Effect of Microstructural Change under Pressure during Isostatic Pressing on Mechanical and Electrical Properties of Isotropic Carbon Blocks
- [Korean]
- Microstructure and Mechanical Property of Ti-Mn-Cu Alloys with Magnetic Pulsed Compaction
- Ye Jun Yun, Chun Woong Park, Won June Choi, Jongmin Byun
- J Korean Powder Metall Inst. 2021;28(1):20-24. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.20
- 832 View
- 6 Download
-
 Abstract
Abstract
 PDF
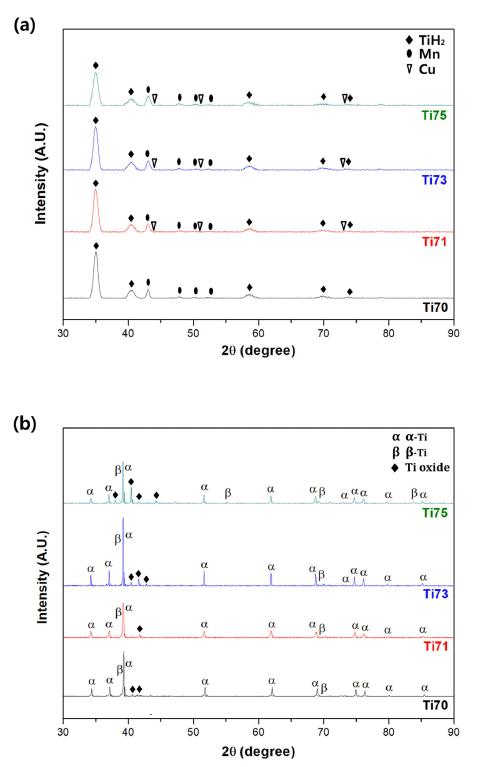
PDF Ti-based alloys are widely used in biomaterials owing to their excellent biocompatibility. In this study, Ti- Mn-Cu alloys are prepared by high-energy ball milling, magnetic pulsed compaction, and pressureless sintering. The microstructure and microhardness of the Ti-Mn-Cu alloys with variation of the Cu addition and compaction pressure are analyzed. The correlation between the composition, compaction pressure, and density is investigated by measuring the green density and sintered density for samples with different compositions, subjected to various compaction pressures. For all compositions, it is confirmed that the green density increases proportionally as the compaction pressure increases, but the sintered density decreases owing to gas formation from the pyrolysis of TiH2 powders and reduction of oxides on the surface of the starting powders during the sintering process. In addition, an increase in the amount of Cu addition changes the volume fractions of the α-Ti and β-Ti phases, and the microstructure of the alloys with different compositions also changes. It is demonstrated that these changes in the phase volume fraction and microstructure are closely related to the mechanical properties of the Ti-Mn-Cu alloys.
- [Korean]
- Analysis of Wafer Cleaning Solution Characteristics and Metal Dissolution Behavior according to the Addition of Chelating Agent
- Myungsuk Kim, Keunhyuk Ryu, Kun-Jae Lee
- J Korean Powder Metall Inst. 2021;28(1):25-30. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.25

- 1,033 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF The surface of silicon dummy wafers is contaminated with metallic impurities owing to the reaction with and adhesion of chemicals during the oxidation process. These metallic impurities negatively affect the device performance, reliability, and yield. To solve this problem, a wafer-cleaning process that removes metallic impurities is essential. RCA (Radio Corporation of America) cleaning is commonly used, but there are problems such as increased surface roughness and formation of metal hydroxides. Herein, we attempt to use a chelating agent (EDTA) to reduce the surface roughness, improve the stability of cleaning solutions, and prevent the re-adsorption of impurities. The bonding between the cleaning solution and metal powder is analyzed by referring to the Pourbaix diagram. The changes in the ionic conductivity, H2O2 decomposition behavior, and degree of dissolution are checked with a conductivity meter, and the changes in the absorbance and particle size before and after the reaction are confirmed by ultraviolet-visible spectroscopy (UV-vis) and dynamic light scattering (DLS) analyses. Thus, the addition of a chelating agent prevents the decomposition of H2O2 and improves the life of the silicon wafer cleaning solution, allowing it to react smoothly with metallic impurities.
- [Korean]
- Evaluation of Oxygen Reduction and Surface Chemical State of Ti-48Al-2Cr-2Nb Powder by Ca Vapor
- Taeheon Kim, Hanjung Kwon, Jae-Won Lim
- J Korean Powder Metall Inst. 2021;28(1):31-37. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.31

- 1,129 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF This study explores reducing the oxygen content of a commercial Ti-48Al-2Cr-2Nb powder to less than 400 ppm by deoxidation in the solid state (DOSS) using Ca vapor, and investigates the effect of Ca vapor on the surface chemical state. As the deoxidation temperature increases, the oxygen concentration of the Ti-48Al-2Cr-2Nb powder decreases, achieving a low value of 745 ppm at 1100°C. When the deoxidation time is increased to 2 h, the oxygen concentration decreases to 320pp m at 1100°C, and the oxygen reduction rate is approximately 78% compared to that of the raw material. The deoxidized Ti-48Al-2Cr-2nb powder maintains a spherical shape, but the surface shape changes slightly owing to the reaction of Ca and Al. The oxidation state of Ti and Al on the surface of the Ti-48Al-2Cr-2Nb powder corresponds to a mixture of TiO2 and Al2O3. As a result, the peaks of metallic Ti and Ti suboxide intensify as TiO2 and Al2O3 in the surface oxide layer are reduced by Ca vapor deposition
-
Citations
Citations to this article as recorded by- Production of spherical TiAl alloy powder by copper-assisted spheroidization
Jin Qian, Bo Yin, Dashun Dong, Geng Wei, Ming Shi, Shaolong Tang
Journal of Materials Research and Technology.2023; 25: 1860. CrossRef - Ca-Mg Multiple Deoxidation of Ti-50Al-2Cr-2Nb Intermetallic Compound Powder for Additive Manufacturing
Seongjae Cho, Taeheon Kim, Jae-Won Lim
ECS Journal of Solid State Science and Technology.2022; 11(4): 045008. CrossRef
- Production of spherical TiAl alloy powder by copper-assisted spheroidization
- [Korean]
- Mn-doping Effect on the Blackness and NIR Reflectance of Fe2O3 Cool Pigments
- Jin Soo Hwang, Kyeong Youl Jung
- J Korean Powder Metall Inst. 2021;28(1):38-43. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.38

- 816 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF A high NIR-reflective black pigment is developed by Mn doping of Fe2O3. The pigment powders are prepared by spray pyrolysis, and the effect of the Mn concentration on the blackness and optical properties is investigated. Mn doping into the crystal lattice of α-Fe2O3 is found to effectively change the powder color from red to black, lowering the NIR reflectance compared to that of pure Fe2O3. The pigment doped with 10% Mn, i.e., Fe1.8Mn0.2O3, exhibits a black color with an optical bandgap of 1.3 eV and a Chroma value of 1.14. The NIR reflectance of the prepared Fe1.8Mn0.2O3 black pigment is 2.2 times higher than that of commercially available carbon black, and this material is proven to effectively work as a cool pigment in a temperature rise experiment under near-infrared illumination.
-
Citations
Citations to this article as recorded by- Enhanced near-infrared reflectance of TiO2/(Fe,Mn)2O3 composite black pigments synthesized by spray pyrolysis
Jin Soo Hwang, Kyeong Youl Jung
Ceramics International.2026; 52(1): 1041. CrossRef
- Enhanced near-infrared reflectance of TiO2/(Fe,Mn)2O3 composite black pigments synthesized by spray pyrolysis
- [Korean]
- Effects of Synthesis Conditions on Luminescence Characteristics of Glutathione Capped ZnSe Nano particles
- Geum Ji Back, Ha Yeon Song, Min Seo Lee, Hyun Seon Hong
- J Korean Powder Metall Inst. 2021;28(1):44-50. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.44
- 1,220 View
- 7 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
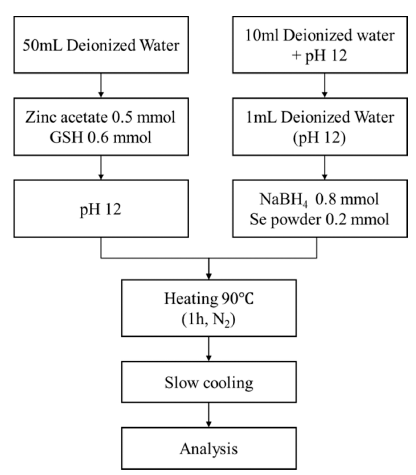
PDF Zinc selenide (ZnSe) nanoparticles were synthesized in aqueous solution using glutathione (GSH) as a ligand. The influence of the ligand content, reaction temperature, and hydroxyl ion concentration (pH) on the fabrication of the ZnSe particles was investigated. The optical properties of the synthesized ZnSe particles were characterized using various analytical techniques. The nanoparticles absorbed UV-vis light in the range of 350-400 nm, which is shorter than the absorption wavelength of bulk ZnSe particles (460 nm). The lowest ligand concentration for achieving good light absorption and emission properties was 0.6 mmol. The reaction temperature had an impact on the emission properties; photoluminescence spectroscopic analysis showed that the photo-discharge characteristics were greatly enhanced at high temperatures. These discharge characteristics were also affected by the hydroxyl ion concentration in solution; at pH 13, sound emission characteristics were observed, even at a low temperature of 25°C. The manufactured nanoparticles showed excellent light absorption and emission properties, suggesting the possibility of fabricating ZnSe QDs in aqueous solutions at low temperatures.
-
Citations
Citations to this article as recorded by- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
Geum Ji Back, Ha Yeon Song, Min Seo Lee, Jaesik Yoon, Hyun Seon Hong
Journal of Crystal Growth.2024; 626: 127475. CrossRef - Effect of UV Irradiation on Optical Properties of Water-Based Synthetic Zinc Selenide Quantum Dots
Geum Ji Back, Yu Jin Kang, I Ju Kang, Jeong Hyeon Lim, Hyun Seon Hong
Korean Journal of Metals and Materials.2022; 60(2): 160. CrossRef
- Green synthesis and luminescence characteristics of ZnSe-ZnS core-shell quantum dots
- [Korean]
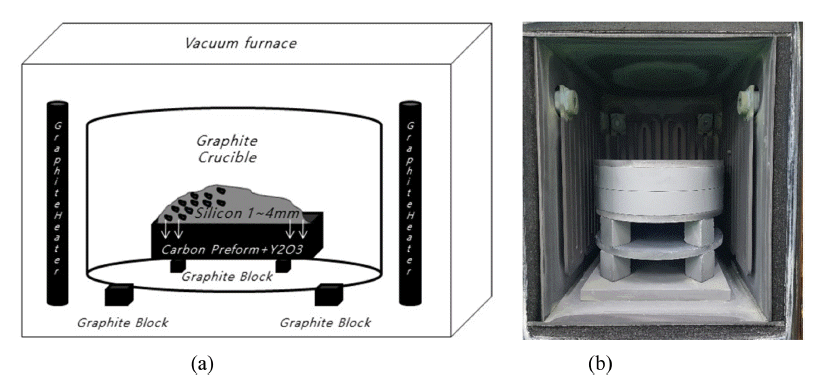
- RBSC Prepared by Si Melt Infiltration into the Y2O3 Added Carbon Preform
- Min-Ho Jang, Kyeong-Sik Cho
- J Korean Powder Metall Inst. 2021;28(1):51-58. Published online February 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.1.51
- 1,451 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF The conversion of carbon preforms to dense SiC by liquid infiltration is a prospectively low-cost and reliable method of forming SiC-Si composites with complex shapes and high densities. Si powder was coated on top of a 2.0wt .% Y2O3-added carbon preform, and reaction bonded silicon carbide (RBSC) was prepared by infiltrating molten Si at 1,450°C for 1-8 h. Reactive sintering of the Y2O3-free carbon preform caused Si to be pushed to one side, thereby forming cracking defects. However, when prepared from the Y2O3-added carbon preform, a SiC-Si composite in which Si is homogeneously distributed in the SiC matrix without cracking can be produced. Using the Si + C → SiC reaction at 1,450°C, 3C and 6H SiC phases, crystalline Si, and Y2O3 were generated based on XRD analysis, without the appearance of graphite. The RBSC prepared from the Y2O3-added carbon preform was densified by increasing the density and decreasing the porosity as the holding time increased at 1,450°C. Dense RBSC, which was reaction sintered at 1,450°C for 4 h from the 2.0wt.% Y2O3-added carbon preform, had an apparent porosity of 0.11% and a relative density of 96.8%.
TOP
 KPMI
KPMI




 First
First Prev
Prev


