Search
- Page Path
- HOME > Search
- [Korean]
- Fabrication of CNT dispersed Cu matrix composites by wet mixing and spark plasma sintering process
- Seungchan Cho, Ilguk Jo, Sang-Bok Lee, Sang-Kwan Lee, Moonhee Choi, Jehong Park, Hansang Kwon, Yangdo Kim
- J Korean Powder Metall Inst. 2018;25(2):158-164. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.158

- 967 View
- 18 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Multi-walled carbon nanotube (MWCNT)–copper (Cu) composites are successfully fabricated by a combination of a binder-free wet mixing and spark plasma sintering (SPS) process. The SPS is performed under various conditions to investigate optimized processing conditions for minimizing the structural defects of CNTs and densifying the MWCNT–Cu composites. The electrical conductivities of MWCNT–Cu composites are slightly increased for compositions containing up to 1 vol.% CNT and remain above the value for sintered Cu up to 2 vol.% CNT. Uniformly dispersed CNTs in the Cu matrix with clean interfaces between the treated MWCNT and Cu leading to effective electrical transfer from the treated MWCNT to the Cu is believed to be the origin of the improved electrical conductivity of the treated MWCNT–Cu composites. The results indicate the possibility of exploiting CNTs as a contributing reinforcement phase for improving the electrical conductivity and mechanical properties in the Cu matrix composites.
-
Citations
Citations to this article as recorded by- Proposing Machine Learning Models Suitable for Predicting Open Data Utilization
Junyoung Jeong, Keuntae Cho
Sustainability.2024; 16(14): 5880. CrossRef
- Proposing Machine Learning Models Suitable for Predicting Open Data Utilization
- [English]
- The Synthesis and Photocatalytic activity of Carbon Nanotube-mixed TiO2 Nanotubes
- Chun Woong Park, Young Do Kim, Tohru Sekino, Se Hoon Kim
- J Korean Powder Metall Inst. 2017;24(4):279-284. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.279
- 1,543 View
- 11 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
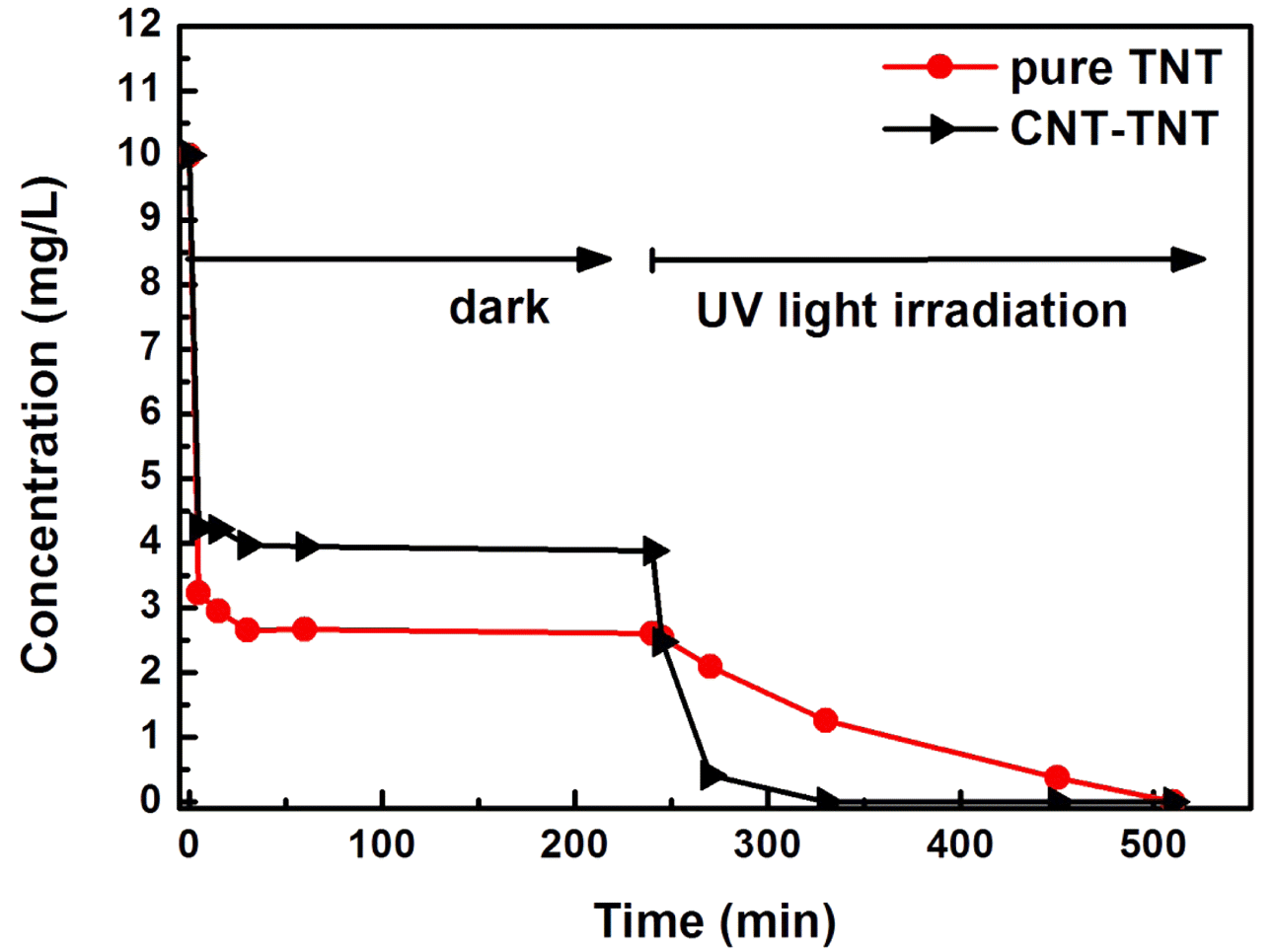
PDF The formation mechanism and photocatalytic properties of a multiwalled carbon nanotube (MWCNT)/TiO2- based nanotube (TNTs) composite are investigated. The CNT/TNT composite is synthesized via a solution chemical route. It is confirmed that this 1-D nanotube composite has a core-shell nanotubular structure, where the TNT surrounds the CNT core. The photocatalytic activity investigated based on the methylene blue degradation test is superior to that of with pure TNT. The CNTs play two important roles in enhancing the photocatalytic activity. One is to act as a template to form the core-shell structure while titanate nanosheets are converted into nanotubes. The other is to act as an electron reservoir that facilitates charge separation and electron transfer from the TNT, thus decreasing the electronhole recombination efficiency.
-
Citations
Citations to this article as recorded by- Carbon-based photocatalysis in organic transformation
Md Razu Ahmed, Yuta Nishina
Bulletin of the Chemical Society of Japan.2026;[Epub] CrossRef - Low-Dimensional Carbon and Titania Nanotube Composites via a Solution Chemical Process and Their Nanostructural and Electrical Properties for Electrochemical Devices
Sunghun Eom, Sung Hun Cho, Tomoyo Goto, Myoung Pyo Chun, Tohru Sekino
ACS Applied Nano Materials.2019; 2(10): 6230. CrossRef
- Carbon-based photocatalysis in organic transformation
- [Korean]
- Evaluation of TiO2 Photocatalytic Activity with Addition of Carbon Nanotube
- In-Chul Yeo, In-Cheol Kang
- J Korean Powder Metall Inst. 2016;23(6):458-465. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.458

- 542 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF A TiO2/CNT nanohybrid photocatalyst is synthesized via sol-gel route, with titanium (IV) isopropoxide and multi-walled carbon nanotubes (MWCNTs) as the starting materials. The microstructures and phase constitution of the nanohybrid TiO2/CNT (0.005wt%) samples after calcination at 450°C, 550°C and 650°C in air are compared with those of pure TiO2 using field-emission scanning electron microscopy and X-ray diffraction, respectively. In addition, the photocatalytic activity of the nanohybrid is compared with that of pure TiO2 with regard to the degradation of methyl orange under visible light irradiation. The TiO2/CNT composite exhibits a fast grain growth and phase transformation during calcination. The nanocomposite shows enhanced photocatalytic activity under visible light irradiation in comparison to pure TiO2 owing to not only better adsorption capability of CNT but also effective electron transfer between TiO2 and CNTs. However, the high calcination temperature of 650°C, regardless of addition of CNT, causes a decrease in photocatalytic activity because of grain growth and phase transformation to rutile. These results such as fast phase transformation to rutile and effective electron transfer are related to carbon doping into TiO2.
- [Korean]
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
- Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2016;23(3):235-239. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.235
- 489 View
- 0 Download
-
 Abstract
Abstract
 PDF
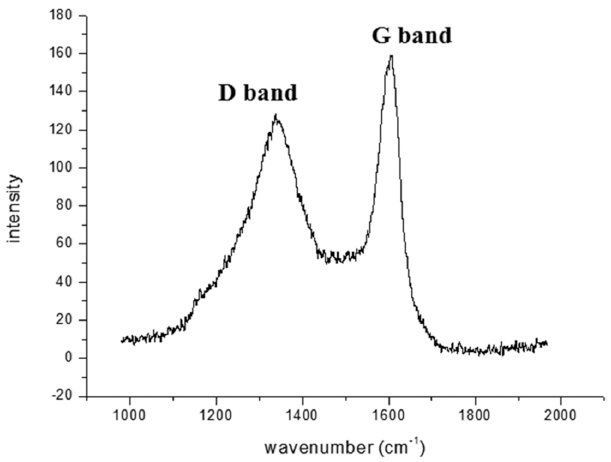
PDF This study is performed to fabricate a Ti porous body by freeze drying process using titanium hydride (TiH2) powder and camphene. Then, the Ti porous body is employed to synthesize carbon nanotubes (CNTs) using thermal catalytic chemical vapor deposition (CCVD) with Fe catalyst and methane (CH4) gas to increase the specific surface area. The synthesized Ti porous body has 100 μm-sized macropores and 10-30 μm-sized micropores. The synthesized CNTs have random directions and are entangled with adjacent CNTs. The CNTs have a bamboo-like structure, and their average diameter is about 50 nm. The Fe nano-particles observed at the tip of the CNTs indicate that the tip growth model is applicable. The specific surface area of the CNT-coated Ti porous body is about 20 times larger than that of the raw Ti porous body. These CNT-coated Ti porous bodies are expected to be used as filters or catalyst supports.
- [English]
- Fabrication and Mechanical Characteristics of Bulk Nickel/Carbon Nanotube Nanocomposites via the Electrical Explosion of Wire in Liquid and Spark Plasma Sintering Method
- Thuyet-Nguyen Minh, Hai-Nguyen Hong, Won Joo Kim, Ho Yoon Kim, Jin-Chun Kim
- J Korean Powder Metall Inst. 2016;23(3):213-220. Published online June 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.3.213
- 1,278 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
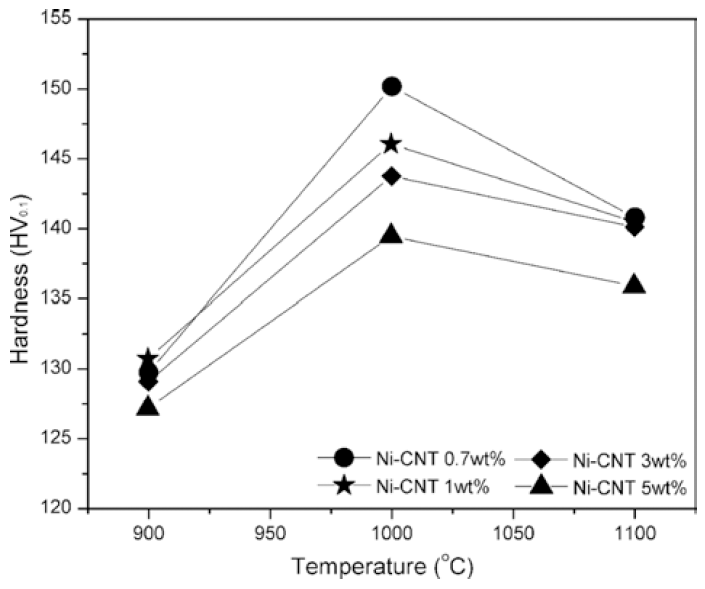
PDF In this study, bulk nickel-carbon nanotube (CNT) nanocomposites are synthesized by a novel method which includes a combination of ultrasonication, electrical explosion of wire in liquid and spark plasma sintering. The mechanical characteristics of the bulk Ni-CNT composites synthesized with CNT contents of 0.7, 1, 3 and 5 wt.% are investigated. X-ray diffraction, optical microscopy and field emission scanning electron microscopy techniques are used to observe the different phases, morphologies and structures of the composite powders as well as the sintered samples. The obtained results reveal that the as-synthesized composite exhibits substantial enhancement in the microhardness and values more than 140 HV are observed. However an empirical reinforcement limit of 3 wt.% is determined for the CNT content, beyond which, there is no significant improvement in the mechanical properties.
-
Citations
Citations to this article as recorded by- Fabrication of nanocomposites by electric explosion of stainless steel capillaries filled with carbon nanotubes
Tao Jiang, Zhongyu Hou
Applied Surface Science.2020; 513: 145824. CrossRef - Effect of a nano-sized TiC particle addition on the flow-assisted corrosion resistance of SA 106B carbon steel
Jin-Ju Park, Eun-Kwang Park, Gyoung-Ja Lee, Chang-Kyu Rhee, Min-Ku Lee
Applied Surface Science.2017; 415: 143. CrossRef
- Fabrication of nanocomposites by electric explosion of stainless steel capillaries filled with carbon nanotubes
- [Korean]
- Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
- Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2015;22(2):122-128. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.122
- 787 View
- 1 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
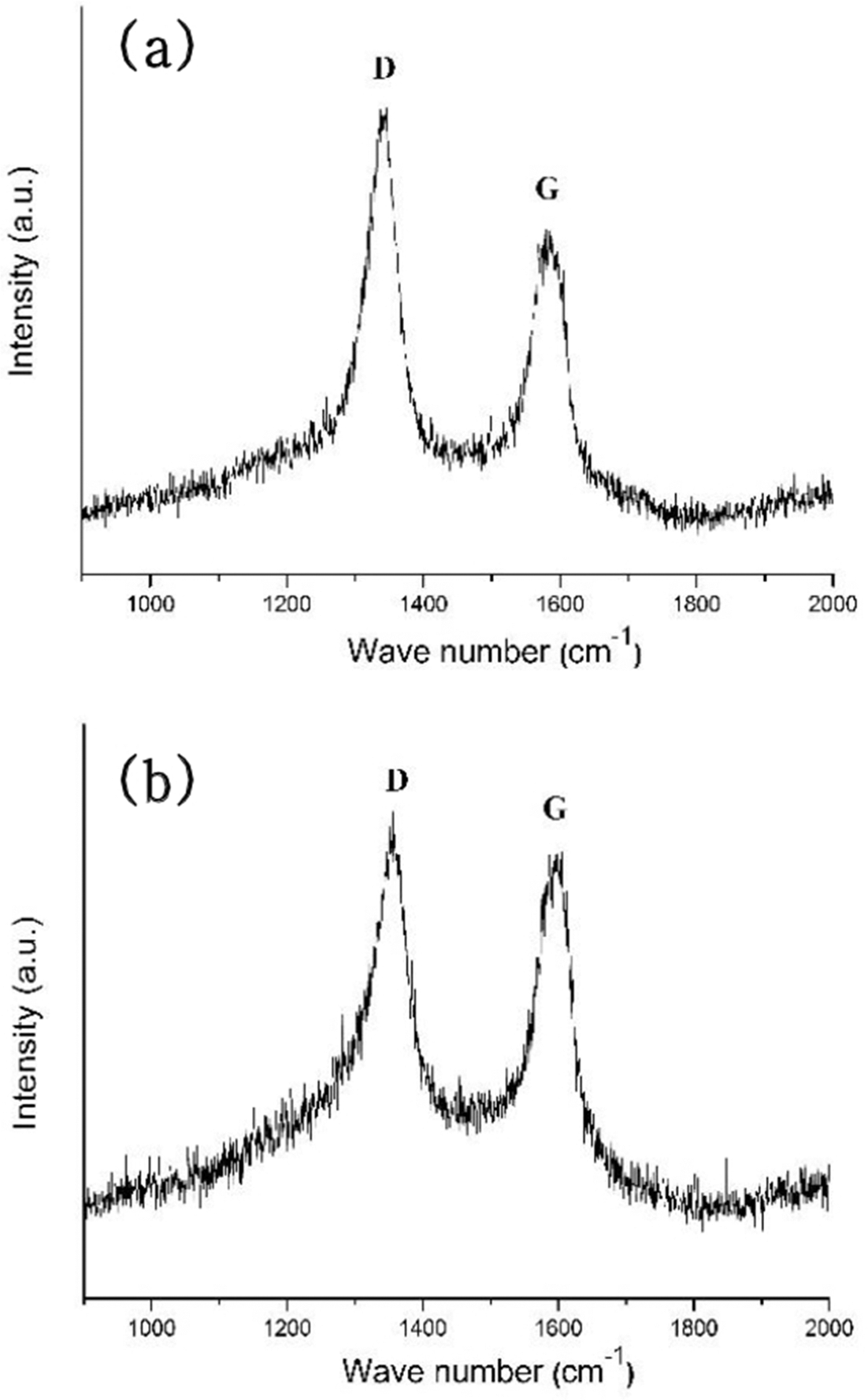
PDF In this study, titanium(Ti) meshes and porous bodies are employed to synthesize carbon nanotubes(CNTs) using methane(CH4) gas and camphene solution, respectively, by chemical vapor deposition. Camphene is impregnated into Ti porous bodies prior to heating in a furnace. Various microscopic and spectroscopic techniques are utilized to analyze CNTs. It is found that CNTs are more densely and homogeneously populated on the camphene impregnated Ti-porous bodies as compared to CNTs synthesized with methane on Ti-porous bodies. It is elucidated that, when synthesized with methane, few CNTs are formed inside of Ti porous bodies due to methane supply limited by internal structures of Ti porous bodies. Ti-meshes and porous bodies are found to be multi-walled with high degree of structural disorders. These CNTs are expected to be utilized as catalyst supports in catalytic filters and purification systems.
-
Citations
Citations to this article as recorded by- Recent progress in additive manufacturing of porous titanium: From design to applications
Haoxin Song, Chen Wang, Wenzheng Yu, Mingsen Zhang, Jinqiang Shao, Hanwen Liang, Tingting Wu, Xiaoxiao Dong
Journal of Alloys and Compounds.2025; 1026: 180451. CrossRef - Solvent induced surface modifications on hydrogen storage performance of ZnO nanoparticle decorated MWCNTs
Madhavi Konni, Anima S. Dadhich, Saratchandra Babu Mukkamala
Sustainable Energy & Fuels.2018; 2(2): 466. CrossRef - Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs
Ye-Ji Han, Soo-Jin Park
Applied Surface Science.2017; 415: 85. CrossRef
- Recent progress in additive manufacturing of porous titanium: From design to applications
- [Korean]
- CNT Growth Behavior on Ti Substrate by Catalytic CVD Process with Temperature Gradient in Tube Furnace
- Ju Hyuk Park, Jong Min Byun, Hyung Soo Kim, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(5):371-376. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.371
- 1,057 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, modified catalytic chemical vapor deposition (CCVD) method was applied to control the CNTs (carbon nanotubes) growth. Since titanium (Ti) substrate and iron (Fe) catalysts react one another and form a new phase (Fe2TiO5) above 700°C, the decrease of CNT yield above 800°C where methane gas decomposes is inevitable under common CCVD method. Therefore, we synthesized CNTs on the Ti substrate by dividing the tube furnace into two sections (left and right) and heating them to different temperatures each. The reactant gas flew through from the end of the right tube furnace while the Ti substrate was placed in the center of the left tube furnace. When the CNT growth temperature was set 700/950°C (left/right), CNTs with high yield were observed. Also, by examining the micro-structure of CNTs of 700/950°C, it was confirmed that CNTs show the bamboo-like structure.
-
Citations
Citations to this article as recorded by- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
Journal of Korean Powder Metallurgy Institute.2016; 23(3): 235. CrossRef - Synthesis of CNT on a Camphene Impregnated Titanium Porous Body by Thermal Chemical Vapor Deposition
Hogyu Kim, Hye Rim Choi, Jong Min Byun, Myung-Jin Suk, Sung-Tag Oh, Young Do Kim
Journal of Korean Powder Metallurgy Institute.2015; 22(2): 122. CrossRef
- Fabrication of Ti Porous body with Improved Specific Surface Area by Synthesis of CNTs
TOP
 KPMI
KPMI


 First
First Prev
Prev


