Search
- Page Path
- HOME > Search
- [Korean]
- Preparation and Characterization of N-doped Na2Ti6O13@TiO2 Composites for Visible Light Activity
- Duk-Hee Lee, Kyung-Soo Park
- J Powder Mater. 2022;29(6):492-498. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.492
- 735 View
- 6 Download
-
 Abstract
Abstract
 PDF
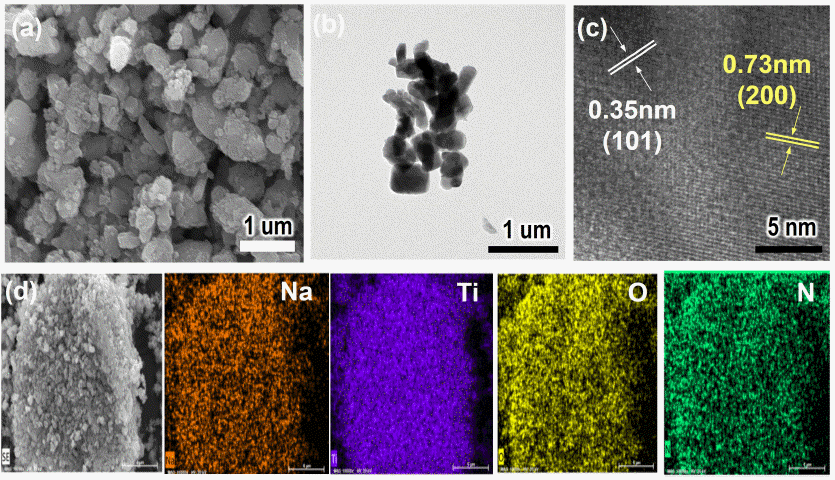
PDF N-doped Na2Ti6O13@TiO2 (denoted as N-NTO@TiO2) composites are successfully synthesized using a simple two-step process: 1) ball-milling of TiO2 with Na2CO3 followed by heat treatment at 900°C; 2) mixing of the prepared Na2Ti6O13 with titanium isopropoxide and calcining with urea at 500°C. The prepared composites are characterized using XRD, SEM, TEM, FTIR, and BET. The N-NTO@TiO2 composites exhibit well-defined crystalline and anatase TiO2 with exposed {101} facets on the external surface. Moreover, dopant N atoms are uniformly distributed over a relatively large area in the lattice of the composites. Under visible light irradiation, ~51% of the aqueous methylene blue is photodegraded by N-NTO@TiO2 composites, which is higher than the values shown by other samples because of the coupling effects of the hybridization of NTO and TiO2, N-doping, and presence of anatase TiO2 with exposed {101} facets.
- [Korean]
- Study on the Preparation of TiO2 3D Nanostructure for Photocatalyst by Wet Chemical Process
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyoung-Tae Park, Kyung-Soo Park
- J Korean Powder Metall Inst. 2020;27(5):381-387. Published online October 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.5.381

- 1,056 View
- 7 Download
-
 Abstract
Abstract
 PDF
PDF In this work, TiO2 3D nanostructures (TF30) were prepared via a facile wet chemical process using ammonium hexafluorotitanate. The synthesized 3D TiO2 nanostructures exhibited well-defined crystalline and hierarchical structures assembled from TiO2 nanorods with different thicknesses and diameters, which comprised numerous small beads. Moreover, the maximum specific surface area of TiO2 3D nanostructures was observed to be 191 m2g-1, with concentration of F ions on the surface being 2 at%. The TiO2 3D nanostructures were tested as photocatalysts under UV irradiation using Rhodamine B solution in order to determine their photocatalytic performance. The TiO2 3D nanostructures showed a higher photocatalytic activity than that of the other TiO2 samples, which was likely associated with the combined effects of a high crystallinity, unique features of the hierarchical structure, a high specific surface area, and the advantage of adsorbing F ions.
- [Korean]
- Recent Research Progress on the Atomic Layer Deposition of Noble Metal Catalysts for Polymer Electrolyte Membrane Fuel Cell
- Jeong Hwan Han
- J Korean Powder Metall Inst. 2020;27(1):63-71. Published online February 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.1.63
- 618 View
- 4 Download
-
 Abstract
Abstract
 PDF
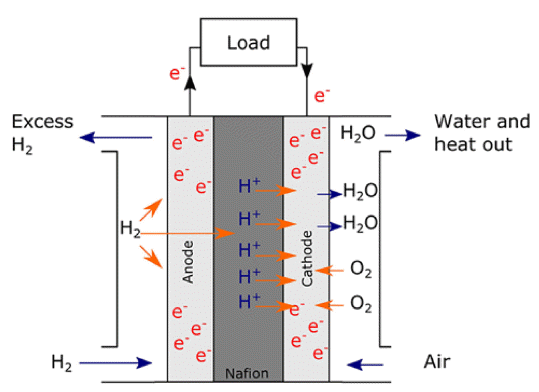
PDF It is necessary to fabricate uniformly dispersed nanoscale catalyst materials with high activity and long-term stability for polymer electrolyte membrane fuel cells with excellent electrochemical characteristics of the oxygen reduction reaction and hydrogen oxidation reaction. Platinum is known as the best noble metal catalyst for polymer electrolyte membrane fuel cells because of its excellent catalytic activity. However, given that Pt is expensive, considerable efforts have been made to reduce the amount of Pt loading for both anode and cathode catalysts. Meanwhile, the atomic layer deposition (ALD) method shows excellent uniformity and precise particle size controllability over the three-dimensional structure. The research progress on noble metal ALD, such as Pt, Ru, Pd, and various metal alloys, is presented in this review. ALD technology enables the development of polymer electrolyte membrane fuel cells with excellent reactivity and durability.
- [Korean]
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
- J Korean Powder Metall Inst. 2018;25(3):257-262. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.257
- 1,154 View
- 9 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
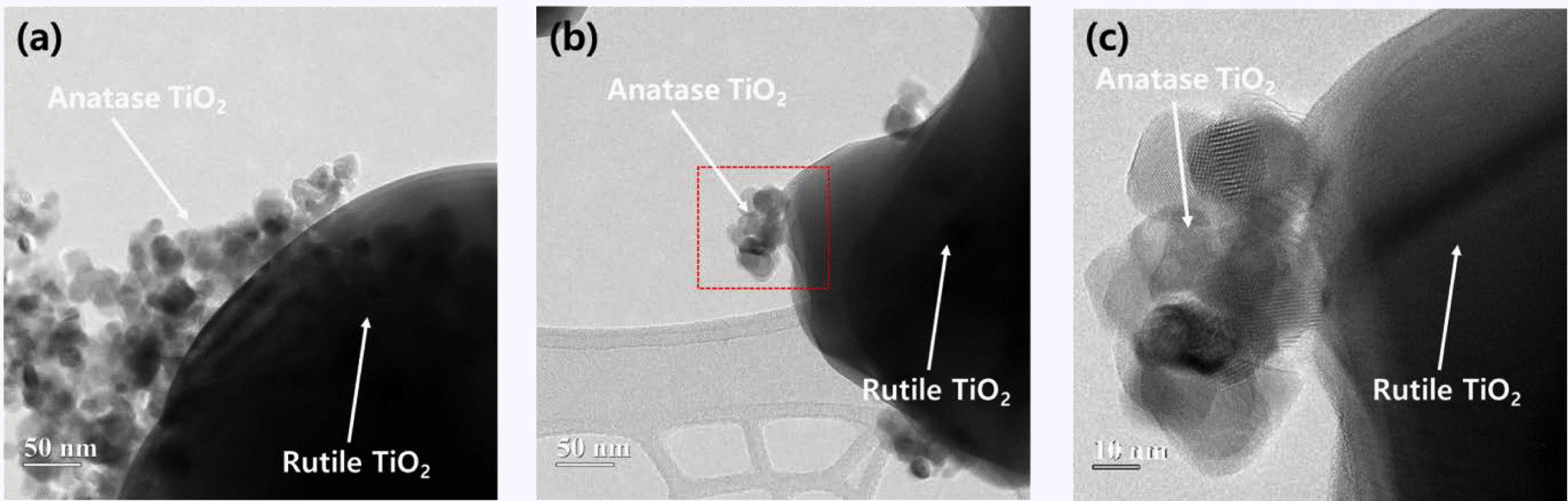
PDF The coupling of two semiconducting materials is an efficient method to improve photocatalytic activity via the suppression of recombination of electron-hole pairs. In particular, the coupling between two different phases of TiO2, i.e., anatase and rutile, is particularly attractive for photocatalytic activity improvement of rutile TiO2 because these coupled TiO2 powders can retain the benefits of TiO2, one of the best photocatalysts. In this study, anatase TiO2 nanoparticles are synthesized and coupled on the surface of rutile TiO2 powders using a microemulsion method and heat treatment. Triton X-100, as a surfactant, is used to suppress the aggregation of anatase TiO2 nanoparticles and disperse anatase TiO2 nanoparticles uniformly on the surface of rutile TiO2 powders. Rutile TiO2 powders coupled with anatase TiO2 nanoparticles are successfully prepared. Additionally, we compare the photocatalytic activity of these rutile-anatase coupled TiO2 powders under ultraviolet (UV) light and demonstrate that the reason for the improvement of photocatalytic activity is microstructural.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 395. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
- [Korean]
- Evaluation of TiO2 Photocatalytic Activity with Addition of Carbon Nanotube
- In-Chul Yeo, In-Cheol Kang
- J Korean Powder Metall Inst. 2016;23(6):458-465. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.458

- 542 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF A TiO2/CNT nanohybrid photocatalyst is synthesized via sol-gel route, with titanium (IV) isopropoxide and multi-walled carbon nanotubes (MWCNTs) as the starting materials. The microstructures and phase constitution of the nanohybrid TiO2/CNT (0.005wt%) samples after calcination at 450°C, 550°C and 650°C in air are compared with those of pure TiO2 using field-emission scanning electron microscopy and X-ray diffraction, respectively. In addition, the photocatalytic activity of the nanohybrid is compared with that of pure TiO2 with regard to the degradation of methyl orange under visible light irradiation. The TiO2/CNT composite exhibits a fast grain growth and phase transformation during calcination. The nanocomposite shows enhanced photocatalytic activity under visible light irradiation in comparison to pure TiO2 owing to not only better adsorption capability of CNT but also effective electron transfer between TiO2 and CNTs. However, the high calcination temperature of 650°C, regardless of addition of CNT, causes a decrease in photocatalytic activity because of grain growth and phase transformation to rutile. These results such as fast phase transformation to rutile and effective electron transfer are related to carbon doping into TiO2.
- [Korean]
- Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
- Hyeong-Chul Kim, Jae-Kil Han
- J Korean Powder Metall Inst. 2016;23(1):33-37. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.33

- 1,019 View
- 3 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF 10 wt.% and 20 wt.%Li-TiO2 composite powders are synthesized by a sol-gel method using titanium isopropoxide and Li2CO3 as precursors. The as-received amorphous 10 wt.%Li-TiO2 composite powders crystallize into the anatase-type crystal structure upon calcination at 450°C, which then changes to the rutile phase at 750°C. The asreceived 20 wt%Li-TiO2 composite powders, on the other hand, crystallize into the anatase-type structure. As the calcination temperature increases, the anatase TiO2 phase gets transformed to the LiTiO2 phase. The peaks for the samples obtained after calcination at 900°C mainly exhibit the LiTiO2 and Li2TiO3 phases. For a comparison of the photocatalytic activity, 10 wt.% and 20 wt.% Li-TiO2 composite powders calcined at 450°C, 600°C, and 750°C are used. The 20 wt.%Li-TiO2 composite powders calcined at 600°C show excellent efficiency for the removal of methylorange.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and characterization of nitrogen-doped TiO2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity
Yifan Zhang, Hye Mee Yang, Soo-Jin Park
Current Applied Physics.2018; 18(2): 163. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
TOP
 KPMI
KPMI


 First
First Prev
Prev


