Search
- Page Path
- HOME > Search
- [English]
- Enhancing the Dispersion Stability of Exfoliated MoS2 Nanoflakes for Na⁺ Intercalation
- Jae Min Sung, Dong-Won Kyung, Ammad Ali, Kee-Ryung Park, Mi Hye Lee, Da-Woon Jeong, Bum Sung Kim, Haejin Hwang, Leeseung Kang, Yoseb Song
- J Powder Mater. 2025;32(5):390-398. Published online October 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00255
- 426 View
- 5 Download
-
 Abstract
Abstract
 PDF
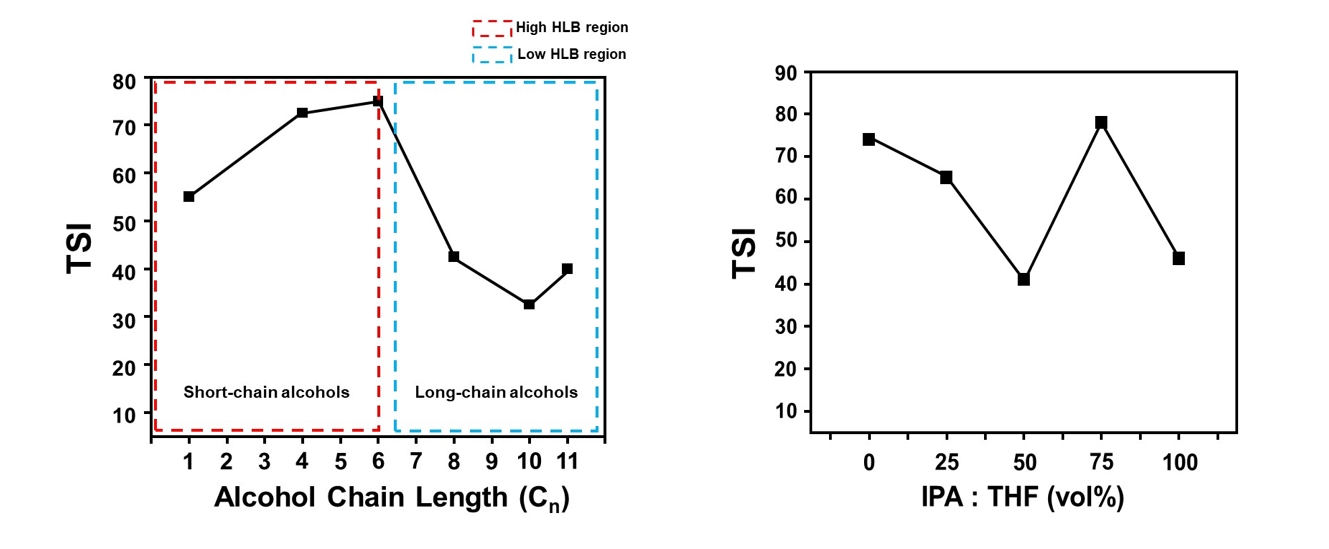
PDF - This study investigated the dispersion stability of exfoliated MoS₂ nanoflakes in various organic solvents and binary mixtures using a Turbiscan optical analyzer. Sedimentation behavior was quantitatively evaluated via transmittance variation (ΔT), backscattering variation (ΔBS), and the Turbiscan stability index (TSI). Alcohol-based solvents were categorized by hydrophilic-lipophilic balance values. Long-chain alcohols, such as 1-undecanol, showed increased stability due to high viscosity and strong hydrophobic affinity with MoS2 basal planes, while short-chain alcohols exhibited poor stabilization. Binary mixtures of isopropanol (IPA) and tetrahydrofuran (THF) were also assessed, with the 5:5 volume ratio showing the best stability profile, including the lowest TSI and minimal ΔT and ΔBS values. This improvement is attributed to synergistic interactions, as IPA stabilizes hydrophilic edge sites, while THF engages with hydrophobic basal surfaces. These findings highlight the importance of balancing physicochemical properties when selecting solvents to improve MoS2 dispersion for structural modification and electrocatalytic applications.
- [Korean]
- Effect of Cellulose Fiber Density Variation on Energy Harvesting Performance in a Hydrovoltaic Generator
- Seung-Hwan Lee, So Hyun Baek, Hyun-Woo Lee, Yongbum Kwon, Kanghyuk Lee, Kee-Ryung Park, Yoseb Song, Bum Sung Kim, Ji Young Park, Yong-Ho Choa, Da-Woon Jeong
- J Powder Mater. 2025;32(2):113-121. Published online April 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00052

- 1,202 View
- 34 Download
-
 Abstract
Abstract
 PDF
PDF - Energy harvesting has become a crucial technology for sustainable energy solutions; in particular, the utilization of ambient water movement in hydrovoltaic generators has emerged as a promising approach. However, optimizing performance requires an understanding of structural factors affecting energy harvesting, particularly capillary effects. This study aimed to improve hydrovoltaic generator performance by adjusting internal fiber density, which influences water transport and ion mobility. Using cold isostatic pressing, cellulose acetate (CA) loading in a urethane mold was varied to optimize internal density. As CA loading increased, the fiber arrangement became denser, narrowing capillary pathways and reducing proton mobility. While open-circuit voltage (VOC) remained stable, short-circuit current (ISC) decreased with higher CA mass. The sample with a loading of 0.3 g exhibited the highest energy harvesting efficiency, achieving ISC = 107.2 μA, VOC = 0.15 V, and power (P) = 16.7 μW. This study provides insights into methods of improving hydrovoltaic generator efficiency through internal structural modifications.
- [Korean]
- A Study on Particle and Crystal Size Analysis of Lithium Lanthanum Titanate Powder Depending on Synthesis Methods (Sol-Gel & Solid-State reaction)
- Jeungjai Yun, Seung-Hwan Lee, So Hyun Baek, Yongbum Kwon, Yoseb Song, Bum Sung Kim, Bin Lee, Rhokyun Kwak, Da-Woon Jeong
- J Powder Mater. 2023;30(4):324-331. Published online August 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.4.324
- 1,312 View
- 15 Download
-
 Abstract
Abstract
 PDF
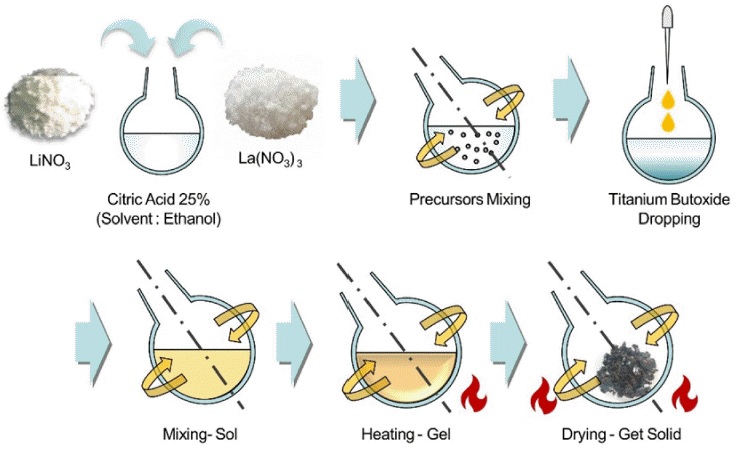
PDF Lithium (Li) is a key resource driving the rapid growth of the electric vehicle industry globally, with demand and prices continually on the rise. To address the limited reserves of major lithium sources such as rock and brine, research is underway on seawater Li extraction using electrodialysis and Li-ion selective membranes. Lithium lanthanum titanate (LLTO), an oxide solid electrolyte for all-solid-state batteries, is a promising Li-ion selective membrane. An important factor in enhancing its performance is employing the powder synthesis process. In this study, the LLTO powder is prepared using two synthesis methods: sol-gel reaction (SGR) and solid-state reaction (SSR). Additionally, the powder size and uniformity are compared, which are indices related to membrane performance. X-ray diffraction and scanning electron microscopy are employed for determining characterization, with crystallite size analysis through the full width at half maximum parameter for the powders prepared using the two synthetic methods. The findings reveal that the powder SGR-synthesized powder exhibits smaller and more uniform characteristics (0.68 times smaller crystal size) than its SSR counterpart. This discovery lays the groundwork for optimizing the powder manufacturing process of LLTO membranes, making them more suitable for various applications, including manufacturing high-performance membranes or mass production of membranes.
- [Korean]
- Research on the Manufacturing Technology for a PDMS Structure-Based Transpiration Generator Using Biomimetic Capillary Phenomenon
- Seung-Hwan Lee, Jeungjai Yun, So Hyun Baek, Yongbum Kwon, Yoseb Song, Bum Sung Kim, Yong-Ho Choa, Da-Woon Jeong
- J Powder Mater. 2023;30(3):268-275. Published online June 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.3.268
- 1,121 View
- 4 Download
-
 Abstract
Abstract
 PDF
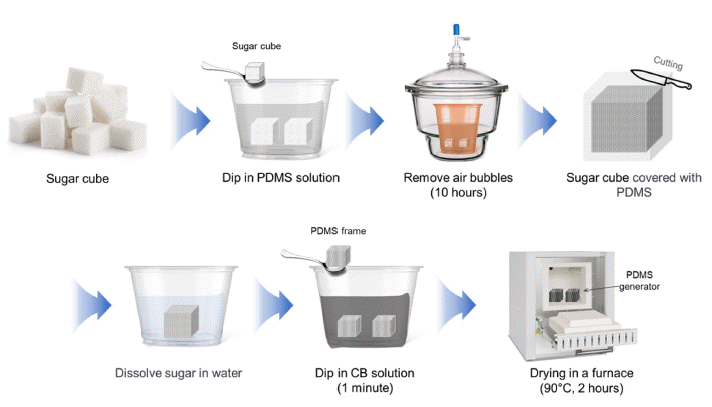
PDF The demand for energy is steadily rising because of rapid population growth and improvements in living standards. Consequently, extensive research is being conducted worldwide to enhance the energy supply. Transpiration power generation technology utilizes the vast availability of water, which encompasses more than 70% of the Earth's surface, offering the unique advantage of minimal temporal and spatial constraints over other forms of power generation. Various principles are involved in water-based energy harvesting. In this study, we focused on explaining the generation of energy through the streaming potential within the generator component. The generator was fabricated using sugar cubes, PDMS, carbon black, CTAB, and DI water. In addition, a straightforward and rapid manufacturing method for the generator was proposed. The PDMS generator developed in this study exhibits high performance with a voltage of 29.6 mV and a current of 8.29 μA and can generate power for over 40h. This study contributes to the future development of generators that can achieve high performance and long-term power generation.
- [Korean]
- Luminescence Properties of InP/ZnS Quantum Dots depending on InP Core synthesis Temperature
- Han Wook Seo, Da-Woon Jeong, Min Young Kim, Seoung Kyun Hyun, Ji Sun On, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(4):321-325. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.321
- 1,440 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
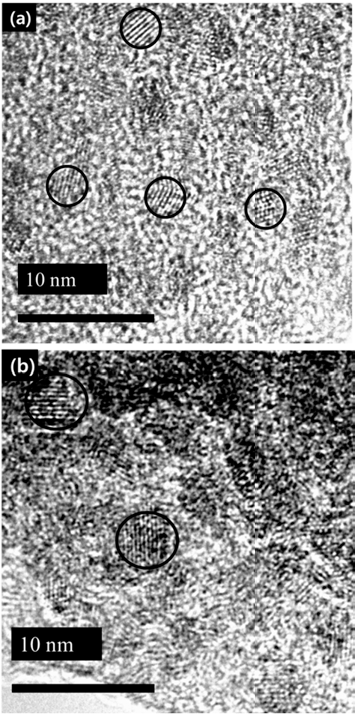
PDF In this study, we investigate the optical properties of InP/ZnS core/shell quantum dots (QDs) by controlling the synthesis temperature of InP. The size of InP determined by the empirical formula tends to increase with temperature: the size of InP synthesized at 140oC and 220oC is 2.46 nm and 4.52 nm, respectively. However, the photoluminescence (PL) spectrum of InP is not observed because of the formation of defects on the InP surface. The growth of InP is observed during the deposition of the shell (ZnS) on the synthesized InP, which is ended up with green-red PL spectrum. We can adjust the PL spectrum and absorption spectrum of InP/ZnS by simply adjusting the core temperature. Thus, we conclude that there exists an optimum shell thickness for the QDs according to the size.
-
Citations
Citations to this article as recorded by- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- [Korean]
- Growth mechanism of InP and InP/ZnS synthesis using colloidal synthesis
- Han wook Seo, Da-woon Jeong, Bin Lee, Seoung kyun Hyun, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(1):6-10. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.6
- 1,427 View
- 5 Download
-
 Abstract
Abstract
 PDF
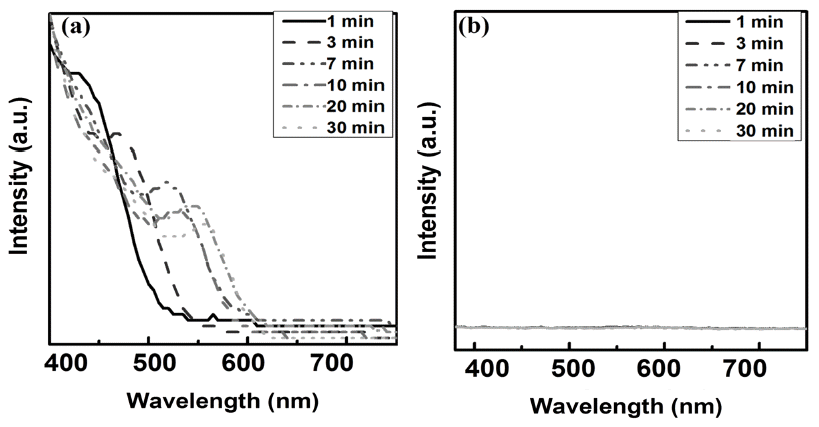
PDF This study investigates the main growth mechanism of InP during InP/ZnS reaction of quantum dots (QDs). The size of the InP core, considering a synthesis time of 1-30 min, increased from the initial 2.56 nm to 3.97 nm. As a result of applying the proposed particle growth model, the migration mechanism, with time index 7, was found to be the main reaction. In addition, after the removal of unreacted In and P precursors from bath, further InP growth (of up to 4.19 nm (5%)), was observed when ZnS was added. The full width at half maximum (FWHM) of the synthesized InP/ZnS quantum dots was found to be relatively uniform, measuring about 59 nm. However, kinetic growth mechanism provides limited information for InP / ZnS core shell QDs, because the surface state of InP changes with reaction time. Further study is necessary, in order to clearly determine the kinetic growth mechanism of InP / ZnS core shell QDs.
- [Korean]
- Surface Treatment Method for Long-term Stability of CdSe/ZnS Quantum Dots
- Hyun-Su Park, Da-Woon Jeong, Bum-Sung Kim, So-Yeong Joo, Chan-Gi Lee, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(1):1-5. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.1
- 1,491 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
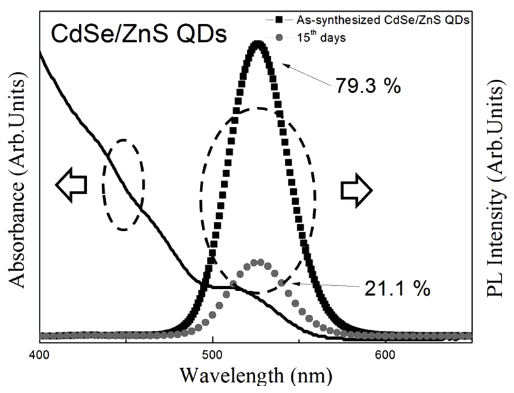
PDF We have investigated the washing method of as-synthesized CdSe/ZnS core/shell structure quantum dots (QDs) and the effective surface passivation method of the washed QDs using PMMA. The quantum yield (QY%) of assynthesized QDs decreases with time, from 79.3% to 21.1%, owing to surface reaction with residual organics. The decreased QY% is restored to the QY% of as-synthesized QDs by washing. However, the QY% of washed QDs also decreases with time, owing to the absence of surface passivation layer. On the other hand, the PMMA-treated QDs maintained a relatively higher QY% after washing than that of the washed QDs that were kept in toluene solution for 30 days. Formation of the PMMA coating layer on CdSe/ZnS QD surface is confirmed by HR-TEM and FT-IR. It is found that the PMMA surface coating, when combined with washing, is useful to be applied in the storage of QDs, owing to its long-term stability.
-
Citations
Citations to this article as recorded by- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
Seung Hwan Ji, Hye Won Yun, Jin Ho Lee, Bum-Sung Kim, Woo-Byoung Kim
Korean Journal of Materials Research.2021; 31(1): 16. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2017; 24(6): 489. CrossRef
- Improvement of Short-Circuit Current of Quantum Dot Sensitive Solar Cell Through Various Size of Quantum Dots
- [Korean]
- The Effect of Surface Defects on the Optical Properties of ZnSe:Eu Quantum Dots
- Da-Woon Jeong, Ji Young Park, Han Wook Seo, Kyoung-Mook Lim, Tae-Yeon Seong, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(5):348-352. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.348
- 1,289 View
- 5 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
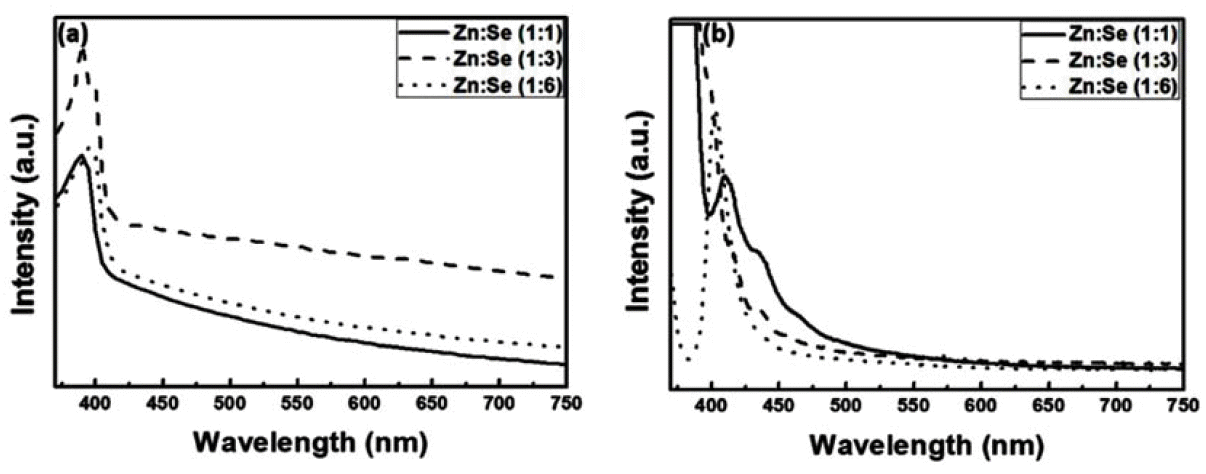
PDF Quantum dots (QDs) are capable of controlling the typical emission and absorption wavelengths because of the bandgap widening effect of nanometer-sized particles. These phosphor particles have been used in optical devices, photovoltaic devices, advanced display devices, and several biomedical complexes. In this study, we synthesize ZnSe QDs with controlled surface defects by a heating-up method. The optical properties of the synthesized particles are analyzed using UV-visible and photoluminescence (PL) measurements. Calculations indicate nearly monodisperse particles with a size of about 5.1 nm at 260°C (full width at half maximum = 27.7 nm). Furthermore, the study results confirm that successful doping is achieved by adding Eu3+ preparing the growth phase of the ZnSe:Eu QDs when heating-up method. Further, we investigate the correlation between the surface defects and the luminescent properties of the QDs.
-
Citations
Citations to this article as recorded by- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
So-Yeong Joo, Hyun-Su Park, Do-yeon Kim, Bum-Sung Kim, Chan Gi Lee, Woo-Byoung Kim
AIP Advances.2018;[Epub] CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- An investigation into the effective surface passivation of quantum dots by a photo-assisted chemical method
- [Korean]
- Optical Characteristics of CdSe/ZnS Quantum Dot with Precursor Flow Rate Synthesized by using Microreactor
- Ji Young Park, Da-Woon Jeong, Won Ju, Han Wook Seo, Yong-Ho Choa, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(2):91-94. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.91
- 1,239 View
- 6 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
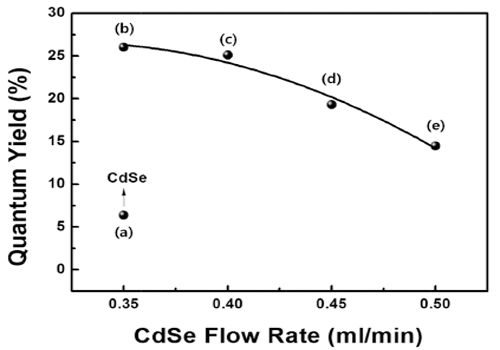
PDF High-quality colloidal CdSe/ZnS (core/shell) is synthesized using a continuous microreactor. The particle size of the synthesized quantum dots (QDs) is a function of the precursor flow rate; as the precursor flow rate increases, the size of the QDs decreases and the band gap energy increases. The photoluminescence properties are found to depend strongly on the flow rate of the CdSe precursor owing to the change in the core size. In addition, a gradual shift in the maximum luminescent wave (λmax) to shorter wavelengths (blue shift) is found owing to the decrease in the QD size in accordance with the quantum confinement effect. The ZnS shell decreases the surface defect concentration of CdSe. It also lowers the thermal energy dissipation by increasing the concentration of recombination. Thus, a relatively high emission and quantum yield occur because of an increase in the optical energy emitted at equal concentration. In addition, the maximum quantum yield is derived for process conditions of 0.35 ml/min and is related to the optimum thickness of the shell material.
-
Citations
Citations to this article as recorded by- Quantum materials made in microfluidics - critical review and perspective
M. Wojnicki, V. Hessel
Chemical Engineering Journal.2022; 438: 135616. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- Quantum materials made in microfluidics - critical review and perspective
- [Korean]
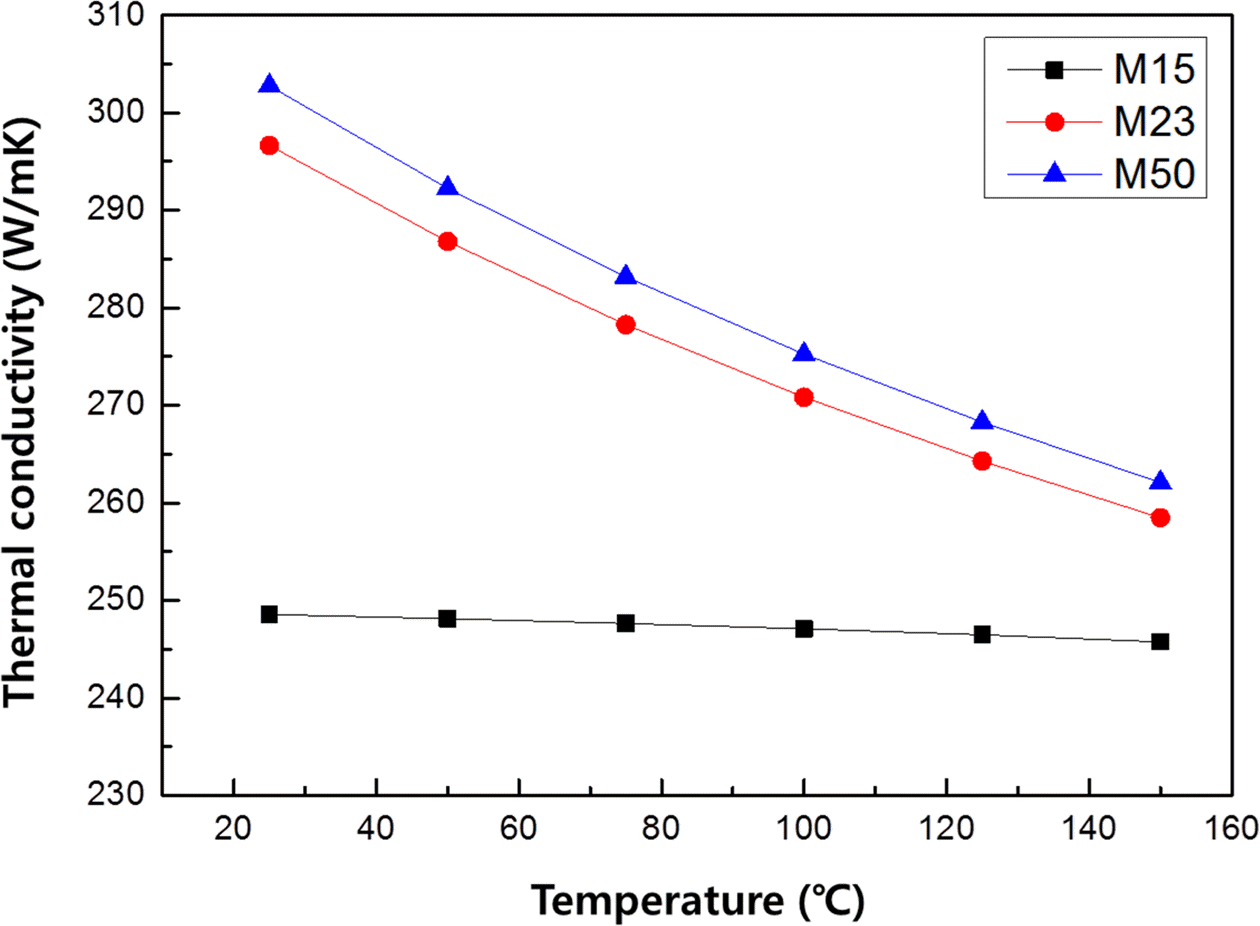
- Thermal Properties of Diamond Aligned Electroless Ni Plating Layer/Oxygen Free Cu Substrates
- Da-Woon Jeong, Song-Yi Kim, Kyoung-Tae Park, Seok-Jun Seo, Taek Soo Kim, Bum Sung Kim
- J Korean Powder Metall Inst. 2015;22(2):134-137. Published online April 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.2.134
- 989 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF The monolayer engineering diamond particles are aligned on the oxygen free Cu plates with electroless Ni plating layer. The mean diamond particle sizes of 15, 23 and 50 μm are used as thermal conductivity pathway for fabricating metal/carbon multi-layer composite material systems. Interconnected void structure of irregular shaped diamond particles allow dense electroless Ni plating layer on Cu plate and fixing them with 37-43% Ni thickness of their mean diameter. The thermal conductivity decrease with increasing measurement temperature up to 150°C in all diamond size conditions. When the diamond particle size is increased from 15 μm to 50 μm (Max. 304 W/mK at room temperature) tended to increase thermal conductivity, because the volume fraction of diamond is increased inside plating layer.
TOP
 KPMI
KPMI


 First
First Prev
Prev


