Search
- Page Path
- HOME > Search
- [Korean]
- Synthesis and Investigation of LiVPO4O1-xFxvia Control of the Fluorine Content for Cathode of Lithium-ion Batteries
- Minkyung Kim, Dong-hee Lee, Changyu Yeo, Sooyeon Choi, Chiwon Choi, Hyunmin Yoon
- J Powder Mater. 2023;30(6):516-520. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.516
- 1,033 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF Highly safe lithium-ion batteries (LIBs) are required for large-scale applications such as electrical vehicles and energy storage systems. A highly stable cathode is essential for the development of safe LIBs. LiFePO4 is one of the most stable cathodes because of its stable structure and strong bonding between P and O. However, it has a lower energy density than lithium transition metal oxides. To investigate the high energy density of phosphate materials, vanadium phosphates were investigated. Vanadium enables multiple redox reactions as well as high redox potentials. LiVPO4O has two redox reactions (V5+/V4+/V3+) but low electrochemical activity. In this study, LiVPO4O is doped with fluorine to improve its electrochemical activity and increase its operational redox potential. With increasing fluorine content in LiVPO4O1-xFx, the local vanadium structure changed as the vanadium oxidation state changed. In addition, the operating potential increased with increasing fluorine content. Thus, it was confirmed that fluorine doping leads to a strong inductive effect and high operating voltage, which helps improve the energy density of the cathode materials.
- [Korean]
- Modulation of Microstructure and Energy Storage Performance in (K,Na)NbO3-Bi(Ni,Ta)O3 Ceramics through Zn Doping
- Jueun Kim, Seonhwa Park, Yuho Min
- J Powder Mater. 2023;30(6):509-515. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.509
- 904 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF Lead-free perovskite ceramics, which have excellent energy storage capabilities, are attracting attention owing to their high power density and rapid charge-discharge speed. Given that the energy-storage properties of perovskite ceramic capacitors are significantly improved by doping with various elements, modifying their chemical compositions is a fundamental strategy. This study investigated the effect of Zn doping on the microstructure and energy storage performance of potassium sodium niobate (KNN)-based ceramics. Two types of powders and their corresponding ceramics with compositions of (1-x)(K,Na)NbO3-xBi(Ni2/3Ta1/3)O3 (KNN-BNT) and (1-x)(K,Na)NbO3-xBi(Ni1/3Zn1/3Ta1/3) O3 (KNN-BNZT) were prepared via solid-state reactions. The results indicate that Zn doping retards grain growth, resulting in smaller grain sizes in Zn-doped KNN-BNZT than in KNN-BNT ceramics. Moreover, the Zn-doped KNNBNZT ceramics exhibited superior energy storage density and efficiency across all x values. Notably, 0.9KNN-0.1BNZT ceramics demonstrate an energy storage density and efficiency of 0.24 J/cm3 and 96%, respectively. These ceramics also exhibited excellent temperature and frequency stability. This study provides valuable insights into the design of KNNbased ceramic capacitors with enhanced energy storage capabilities through doping strategies.
- [Korean]
- Preparation and Characterization of N-doped Na2Ti6O13@TiO2 Composites for Visible Light Activity
- Duk-Hee Lee, Kyung-Soo Park
- J Powder Mater. 2022;29(6):492-498. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.492
- 734 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF N-doped Na2Ti6O13@TiO2 (denoted as N-NTO@TiO2) composites are successfully synthesized using a simple two-step process: 1) ball-milling of TiO2 with Na2CO3 followed by heat treatment at 900°C; 2) mixing of the prepared Na2Ti6O13 with titanium isopropoxide and calcining with urea at 500°C. The prepared composites are characterized using XRD, SEM, TEM, FTIR, and BET. The N-NTO@TiO2 composites exhibit well-defined crystalline and anatase TiO2 with exposed {101} facets on the external surface. Moreover, dopant N atoms are uniformly distributed over a relatively large area in the lattice of the composites. Under visible light irradiation, ~51% of the aqueous methylene blue is photodegraded by N-NTO@TiO2 composites, which is higher than the values shown by other samples because of the coupling effects of the hybridization of NTO and TiO2, N-doping, and presence of anatase TiO2 with exposed {101} facets.
- [Korean]
- Aqueous Synthesis and Luminescent Characteristics of Cu:ZnSe Quantum Dots by Internal Doping Method
- Geum Ji Back, Hyun Seon Hong
- J Powder Mater. 2022;29(5):370-375. Published online October 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.5.370
- 862 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Cu-doped ZnSe quantum dots were successfully synthesized in an aqueous solution using an internal doping method. The effects of ligand type, CuSe synthesis temperature, and heating time on Cu-doped ZnSe synthesis were systematically investigated. Of MPA, GSH, TGA, and NAC used as ligands, MPA was the optimal ligand as determined by PL spectrum analysis. In addition, the emission wavelength was found to depend on the synthesis temperature of the internal doping core of CuSe. As the temperature increased, the doping of Cu2+ was enhanced, and the emission wavelength band was redshifted; accordingly, the emission peaks moved from blue to green (up to 550 nm). Thus, the synthesis of Cu:ZnSe using internal doping in aqueous solutions is a potential method for ecomanufacturing of colortuned ZnSe quantum dots for display applications.
-
Citations
Citations to this article as recorded by- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
Hyun Seon Hong, Yerin Kim, Jea Hyung Kim, Hyeon Seon Ryu, Dahye Song
Journal of the Korean Ceramic Society.2025; 62(3): 472. CrossRef
- Synthesis and luminescence characteristics of manganese-doped ZnSe quantum dots synthesized in aqueous solution through internal doping
- [Korean]
- Photoluminescence Enhancement of Y2O3:Eu3+ Red Phosphor Prepared by Spray Pyrolysis using Aliovalent Cation Substitution and Organic Additives
- Byeong Ho Min, Kyeong Youl Jung
- J Korean Powder Metall Inst. 2020;27(2):146-153. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.146
- 704 View
- 5 Download
-
 Abstract
Abstract
 PDF
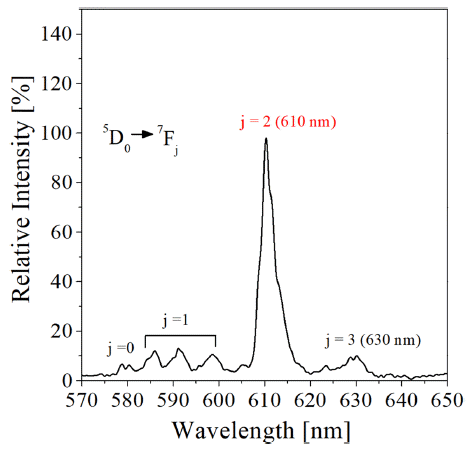
PDF The co-doping effect of aliovalent metal ions such as Mg2+, Ca2+, Sr2+, Ba2+, and Zn2+ on the photoluminescence of the Y2O3:Eu3+ red phosphor, prepared by spray pyrolysis, is analyzed. Mg2+ metal doping is found to be helpful for enhancing the luminescence of Y2O3:Eu3+. When comparing the luminescence intensity at the optimum doping level of each Mg2+ ion, the emission enhancement shows the order of Zn2+ ≈ Ba2+ > Ca2+ > Sr3+> Mg2+. The highest emission occurs when doping approximately 1.3% Zn2+, which is approximately 127% of the luminescence intensity of pure Y2O3:Eu3+. The highest emission was about 127% of the luminescence intensity of pure Y2O3:Eu3+ when doping about 1.3% Zn2+. It is determined that the reason (Y, M)2O3:Eu3+ has improved luminescence compared to that of Y2O3:Eu3+ is because the crystallinity of the matrix is improved and the non-luminous defects are reduced, even though local lattice strain is formed by the doping of aliovalent metal. Further improvement of the luminescence is achieved while reducing the particle size by using Li2CO3 as a flux with organic additives.
- [Korean]
- Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
- Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2019;26(1):49-57. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.49
- 2,009 View
- 22 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
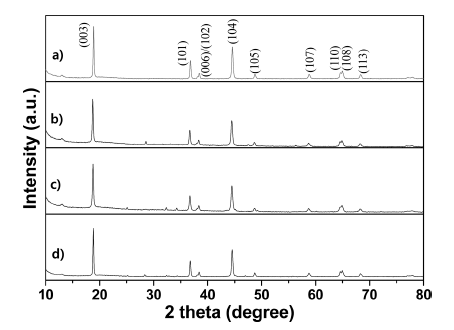
PDF Layered LiNi0.83Co0.11Mn0.06O2 cathode materials single- and dual-doped by the rare-earth elements Ce and Nd are successfully fabricated by using a coprecipitation-assisted solid-phase method. For comparison purposes, nondoping pristine LiNi0.83Co0.11Mn0.06O2 cathode material is also prepared using the same method. The crystal structure, morphology, and electrochemical performances are characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometer (EDS) mapping, and electrochemical techniques. The XRD data demonstrates that all prepared samples maintain a typical α-NaFeO2-layered structure with the
R-3m -
Citations
Citations to this article as recorded by- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
Heewon Choi, Nam-gyu Lim, Seong Jun Lee, Jungsoo Park
Journal of Mechanical Science and Technology.2021; 35(6): 2697. CrossRef - Synthesis of CeVO4-V2O5 nanowires by cation-exchange method for high-performance lithium-ion battery electrode
Xueliu Xu, Shiying Chang, Taofang Zeng, Yidan Luo, Dong Fang, Ming Xie, Jianhong Yi
Journal of Alloys and Compounds.2021; 887: 161237. CrossRef
- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
- [Korean]
- Preparation and Characterization of Visible Light-Sensitive N-doped TiO2 Using a Sol-gel Method
- NaRi Lee, Ri Yu, Tae Kwan Kim, Jae-Hwan Pee, YooJin Kim
- J Korean Powder Metall Inst. 2017;24(6):477-482. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.477

- 1,185 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF Nitrogen-doped titanium dioxide (N-doped TiO2) is attracting continuously increasing attention as a material for environmental photocatalysis. The N-atoms can occupy both interstitial and substitutional positions in the solid, with some evidence of a preference for interstitial sites. In this study, N-doped TiO2 is prepared by the sol–gel method using NH4OH and NH4Cl as N ion doping agents, and the physical and photocatalytic properties with changes in the synthesis temperature and amount of agent are analyzed. The photocatalytic activities of the N-doped TiO2 samples are evaluated based on the decomposition of methylene blue (MB) under visible-light irradiation. The addition of 5 wt% NH4Cl produces the best physical properties. As per the UV-vis analysis results, the N-doped TiO2 exhibits a higher visible-light activity than the undoped TiO2. The wavelength of the N-doped TiO2 shifts to the visible-light region up to 412 nm. In addition, this sample shows MB removal of approximately 81%, with the whiteness increasing to +97 when the synthesis temperature is 600oC. The coloration and phase structure of the N-doped TiO2 are characterized in detail using UV-vis, CIE
Lab color parameter measurements, and powder X-ray diffraction (XRD).
- [Korean]
- Synthesis of Nitrogen-doped Carbon Nanofibers for Oxygen Reduction Reaction
- Geon-Hyoung An, Eun-Hwan Lee, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2016;23(6):420-425. Published online December 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.6.420

- 647 View
- 1 Download
-
 Abstract
Abstract
 PDF
PDF N-doped carbon nanofibers as catalysts for oxygen-reduction reactions are synthesized using electrospinning and carbonization. Their morphologies, structures, chemical bonding states, and electrochemical performance are characterized. The optimized N-doped carbon nanofibers exhibit graphitization of carbon nanofibers and an increased nitrogen doping as well as a uniform network structure. In particular, the optimized N-doped carbon nanofibers show outstanding catalytic activity for oxygen-reduction reactions, such as a half-wave potential (E1/2) of 0.43 V, kinetic limiting current density of 6.2 mA cm-2, electron reduction pathways (n = 3.1), and excellent long-term stability after 2000 cycles, resulting in a lower E1/2 potential degradation of 13 mV. The improvement in the electrochemical performance results from the synergistic effect of the graphitization of carbon nanofibers and the increased amount of nitrogen doping.
TOP
 KPMI
KPMI


 First
First Prev
Prev


