Search
- Page Path
- HOME > Search
- [English]
- A Review of Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries: Challenges and Progress
- Seul Ki Choi, Jaehun Han, Gi Jeong Kim, Yeon Hee Kim, Jaewon Choi, MinHo Yang
- J Powder Mater. 2024;31(4):293-301. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00206
- 11,278 View
- 268 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
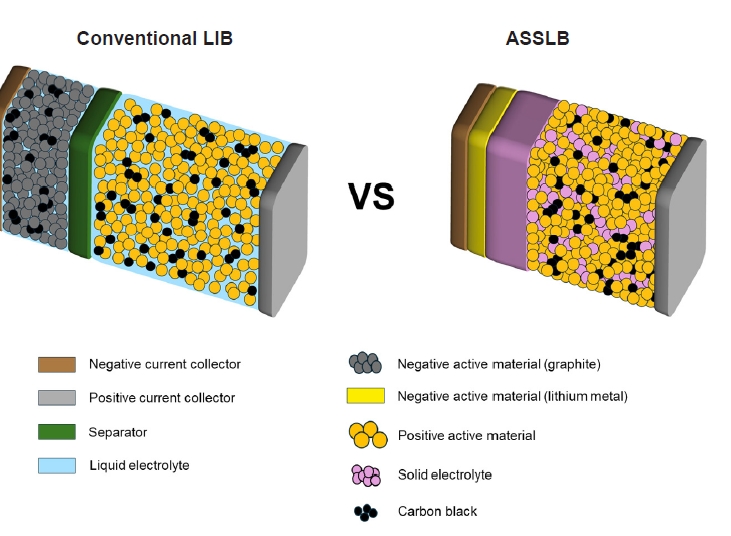
PDF - All-solid-state lithium batteries (ASSLBs) are receiving attention as a prospective next-generation secondary battery technology that can reduce the risk of commercial lithium-ion batteries by replacing flammable organic liquid electrolytes with non-flammable solid electrolytes. The practical application of ASSLBs requires developing robust solid electrolytes that possess ionic conductivity at room temperature on a par with that of organic liquids. These solid electrolytes must also be thermally and chemically stable, as well as compatible with electrode materials. Inorganic solid electrolytes, including oxide and sulfide-based compounds, are being studied as promising future candidates for ASSLBs due to their higher ionic conductivity and thermal stability than polymer electrolytes. Here, we present the challenges currently facing the development of oxide and sulfide-based solid electrolytes, as well as the research efforts underway aiming to resolve these challenges.
-
Citations
Citations to this article as recorded by- A facile synthesis of bulk LiPON in solution for solid-state electrolytes
Osma J. Gomez, Adam Antar, Alex T. Hall, Leopoldo Tapia-Aracayo, Joshua Seo, Nam Kim, Zihan Sun, Ryan Lim, Fu Chen, Yue Li, John Cumings, Gary Rubloff, Sang Bok Lee, David Stewart, Yang Wang
Journal of Materials Chemistry A.2025; 13(34): 28368. CrossRef - Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef - Garnet-type LLZO electrolytes for solid-state lithium batteries: Interfaces, conductivity, in-situ processing, and industrial prospects
Kaleab Habtamu Ayalew, Nithyadharseni Palaniyandy, Mkhulu K. Mathe, Phumlani F. Msomi
Chemical Engineering Journal.2025; 524: 168098. CrossRef
- A facile synthesis of bulk LiPON in solution for solid-state electrolytes
- [Korean]
- Effect of Chelating Agent on Li1.5Al0.5Ti1.5(PO4)3 Particles by Sol-gel Method and Densification
- SungJoon Ryu, Seul Ki Choi, Jong Ho Won, MinHo Yang
- J Powder Mater. 2023;30(5):394-401. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.394
- 1,721 View
- 48 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
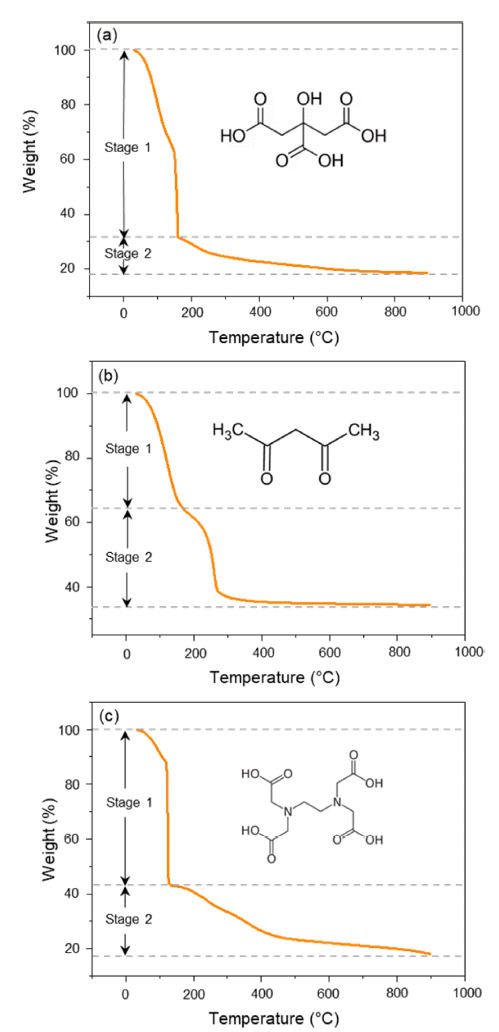
PDF Li1.5Al0.5Ti1.5(PO4)3 (LATP) is considered to be one of the promising solid-state electrolytes owing to its excellent chemical and thermal stability, wide potential range (~5.0 V), and high ionic conductivity (~10-4 S/cm). LATP powders are typically prepared via the sol-gel method by adding and mixing nitrate or alkoxide precursors with chelating agents. Here, the thermal properties, crystallinity, density, particle size, and distribution of LATP powders based on chelating agents (citric acid, acetylacetone, EDTA) are compared to find the optimal conditions for densely sintered LATP with high purity. In addition, the three types of LATP powders are utilized to prepare sintered solid electrolytes and observe the microstructure changes during the sintering process. The pyrolysis onset temperature and crystallization temperature of the powder samples are in the order AC-LATP > CA-LATP > ED-LATP, and the LATP powder utilizing citric acid exhibits the highest purity, as no secondary phase other than LiTi2PO4 phase is observed. LATP with citric acid and acetylacetone has a value close to the theoretical density (2.8 g/cm3) after sintering. In comparison, LATP with EDTA has a low sintered density (2.2 g/cm3) because of the generation of many pores after sintering.
-
Citations
Citations to this article as recorded by- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef
- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
- [Korean]
- A Study on the Microstructures and Ionic Conductivity of Li1.3Al0.3Ti1.7(PO4)3 with Different Synthesis Routes
- Seul Ki Choi, Jeawon Choi, MinHo Yang
- J Powder Mater. 2023;30(2):107-115. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.107
- 2,073 View
- 46 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
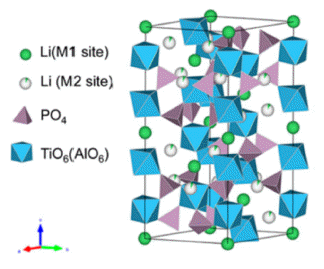
PDF Li1.3Al0.3Ti1.7(PO4)3(LATP) is considered a promising material for all-solid-state lithium batteries owing to its high moisture stability, wide potential window (~6 V), and relatively high ion conductivity (10-3–10-4 S/cm). Solid electrolytes based on LATP are manufactured via sintering, using LATP powder as the starting material. The properties of the starting materials depend on the synthesis conditions, which affect the microstructure and ionic conductivity of the solid electrolytes. In this study, we synthesize the LATP powder using sol-gel and co-precipitation methods and characterize the physical properties of powder, such as size, shape, and crystallinity. In addition, we have prepared a disc-shaped LATP solid electrolyte using LATP powder as the starting material. In addition, X-ray diffraction, scanning electron microscopy, and electrochemical impedance spectroscopic measurements are conducted to analyze the grain size, microstructures, and ion conduction properties. These results indicate that the synthesis conditions of the powder are a crucial factor in creating microstructures and affecting the conduction properties of lithium ions in solid electrolytes.
-
Citations
Citations to this article as recorded by- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
Gwanhee Song, Bojoong Kim, Inkook Hwang, Jiwon Kim, Jinmo Kim, Chang-Bun Yoon
Materials.2025; 18(3): 609. CrossRef
- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
- [Korean]
- The Synthesis of Lithium Lanthanum Titanium Oxide for Solid Electrolyte via Ultrasonic Spray Pyrolysis
- Jaeseok Roh, MinHo Yang, Kun-Jae Lee
- J Powder Mater. 2022;29(6):485-491. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.485

- 1,430 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF Lithium lanthanum titanium oxide (LLTO) is a promising ceramic electrolyte because of its high ionic conductivity at room temperature, low electrical conductivity, and outstanding physical properties. Several routes for the synthesis of bulk LLTO are known, in particular, solid-state synthesis and sol-gel method. However, the extremely low ionic conductivity of LLTO at grain boundaries is one of the major problems for practical applications. To diminish the grain boundary effect, the structure of LLTO is tuned to nanoscale morphology with structures of different dimensionalities (0D spheres, and 1D tubes and wires); this strategy has great potential to enhance the ion conduction by intensifying Li diffusion and minimizing the grain boundary resistance. Therefore, in this work, 0D spherical LLTO is synthesized using ultrasonic spray pyrolysis (USP). The USP method primarily yields spherical particles from the droplets generated by ultrasonic waves passed through several heating zones. LLTO is synthesized using USP, and the effects of each precursor and their mechanisms as well as synthesis parameters are analyzed and discussed to optimize the synthesis. The phase structure of the obtained materials is analyzed using X-ray diffraction, and their morphology and particle size are analyzed using field-emission scanning electron microscopy.
- [Korean]
- Recent Research Progress on the Atomic Layer Deposition of Noble Metal Catalysts for Polymer Electrolyte Membrane Fuel Cell
- Jeong Hwan Han
- J Korean Powder Metall Inst. 2020;27(1):63-71. Published online February 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.1.63
- 569 View
- 4 Download
-
 Abstract
Abstract
 PDF
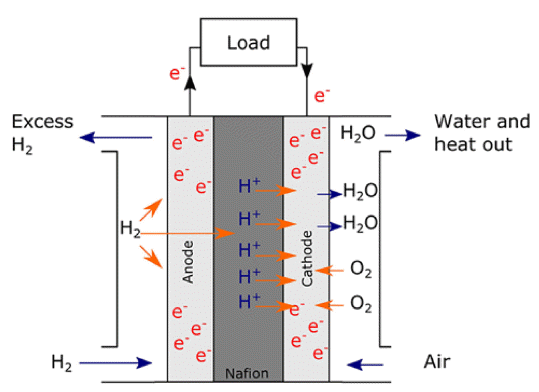
PDF It is necessary to fabricate uniformly dispersed nanoscale catalyst materials with high activity and long-term stability for polymer electrolyte membrane fuel cells with excellent electrochemical characteristics of the oxygen reduction reaction and hydrogen oxidation reaction. Platinum is known as the best noble metal catalyst for polymer electrolyte membrane fuel cells because of its excellent catalytic activity. However, given that Pt is expensive, considerable efforts have been made to reduce the amount of Pt loading for both anode and cathode catalysts. Meanwhile, the atomic layer deposition (ALD) method shows excellent uniformity and precise particle size controllability over the three-dimensional structure. The research progress on noble metal ALD, such as Pt, Ru, Pd, and various metal alloys, is presented in this review. ALD technology enables the development of polymer electrolyte membrane fuel cells with excellent reactivity and durability.
- [English]
- Representative Volume Element Analysis of Fluid-Structure Interaction Effect on Graphite Powder Based Active Material for Lithium-Ion Batteries
- Jin Chul Yun, Seong Jin Park
- J Korean Powder Metall Inst. 2017;24(1):17-23. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.17

- 670 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF In this study, a finite element analysis approach is proposed to predict the fluid-structure interaction behavior of active materials for lithium-ion batteries (LIBs), which are mainly composed of graphite powder. The porous matrix of graphite powder saturated with fluid electrolyte is considered a representative volume element (RVE) model. Three different RVE models are proposed to consider the uncertainty of the powder shape and the porosity. Pwave modulus from RVE solutions are analyzed based on the microstructure and the interaction between the fluid and the graphite powder matrix. From the results, it is found that the large surface area of the active material results in low mechanical properties of LIB, which leads to poor structural durability when subjected to dynamic loads. The results obtained in this study provide useful information for predicting the mechanical safety of a battery pack.
- [Korean]
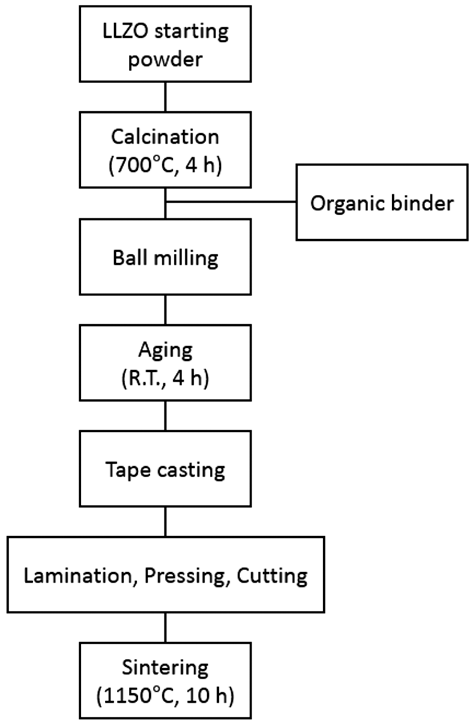
- Fabrication of Solid State Electrolyte Li7La3Zr2O12 thick Film by Tape Casting
- Ran-Hee Shin, Samick Son, Sung-Soo Ryu, Hyung-Tae Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2016;23(5):379-383. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.379
- 1,019 View
- 11 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF A thick film of Li7La3Zr2O12 (LLZO) solid-state electrolyte is fabricated using the tape casting process and is compared to a bulk specimen in terms of the density, microstructure, and ion conductivity. The final thickness of LLZO film after sintering is 240 μm which is stacked up with four sheets of LLZO green films including polymeric binders. The relative density of the LLZO film is 83%, which is almost the same as that of the bulk specimen. The ion conductivity of a LLZO thick film is 2.81 × 10−4 S/cm, which is also similar to that of the bulk specimen, 2.54 × 10−4 S/ cm. However, the microstructure shows a large difference in the grain size between the thick film and the bulk specimen. Although the grain boundary area is different between the thick film and the bulk specimen, the fact that both the ion conductivities are very similar means that no secondary phase exists at the grain boundary, which is thought to originate from nonstoichiometry or contamination.
-
Citations
Citations to this article as recorded by- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
Vivien Kiyek, Martin Hilger, Melanie Rosen, Jürgen Peter Gross, Markus Mann, Dina Fattakhova-Rohlfing, Ruth Schwaiger, Martin Finsterbusch, Olivier Guillon
Journal of Power Sources.2024; 609: 234709. CrossRef - Powder Aerosol Deposition as a Method to Produce Garnet‐Type Solid Ceramic Electrolytes: A Study on Electrochemical Film Properties and Industrial Applications
Tobias Nazarenus, Yanyan Sun, Jörg Exner, Jaroslaw Kita, Ralf Moos
Energy Technology.2021;[Epub] CrossRef - Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
- [Korean]
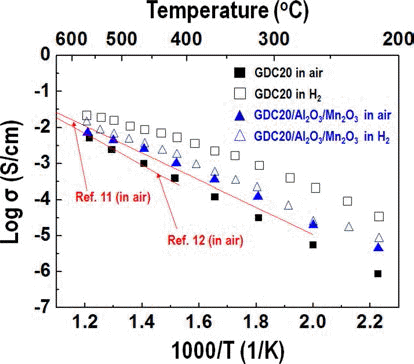
- Synthesis and Characterization of a Ceria Based Composite Electrolyte for Solid Oxide Fuel Cells by an Ultrasonic Spray Pyrolysis Process
- Young-In Lee, Yong-Ho Choa
- J Korean Powder Metall Inst. 2014;21(3):222-228. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.222
- 837 View
- 1 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Much research into fuel cells operating at a temperature below 800°C. is being performed. There are significant efforts to replace the yttria-stabilized zirconia electrolyte with a doped ceria electrolyte that has high ionic conductivity even at a lower temperature. Even if the doped ceria electrolyte has high ionic conductivity, it also shows high electronic conductivity in a reducing environment, therefore, when used as a solid electrolyte of a fuel cell, the powergeneration efficiency and mechanical properties of the fuel cell may be degraded. In this study, gadolinium-doped ceria nanopowder with Al2O3 and Mn2O3 as a reinforcing and electron trapping agents were synthesized by ultrasonic pyrolysis process. After firing, their microstructure and mechanical and electrical properties were investigated and compared with those of pure gadolinium-doped ceria specimen.
-
Citations
Citations to this article as recorded by- High growth-rate atomic layer deposition process of cerium oxide thin film for solid oxide fuel cell
Jin-Geun Yu, Byung Chan Yang, Jeong Woo Shin, Sungje Lee, Seongkook Oh, Jae-Ho Choi, Jaehack Jeong, Wontae Noh, Jihwan An
Ceramics International.2019; 45(3): 3811. CrossRef - Atomic-layer-deposited ZrO2-doped CeO2 thin film for facilitating oxygen reduction reaction in solid oxide fuel cell
Byung Chan Yang, Dohyun Go, Seongkook Oh, Jeong Woo Shin, Hyong June Kim, Jihwan An
Applied Surface Science.2019; 473: 102. CrossRef
- High growth-rate atomic layer deposition process of cerium oxide thin film for solid oxide fuel cell
TOP
 KPMI
KPMI


 First
First Prev
Prev


