Search
- Page Path
- HOME > Search
- [Korean]
- Hydrogen Reduction Behavior of NCM-based Lithium-ion Battery Cathode Materials
- So-Yeong Lee, So-Yeon Lee, Dae-Hyeon Lee, Ho-Sang Sohn
- J Powder Mater. 2024;31(2):163-168. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00017
- 1,422 View
- 38 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
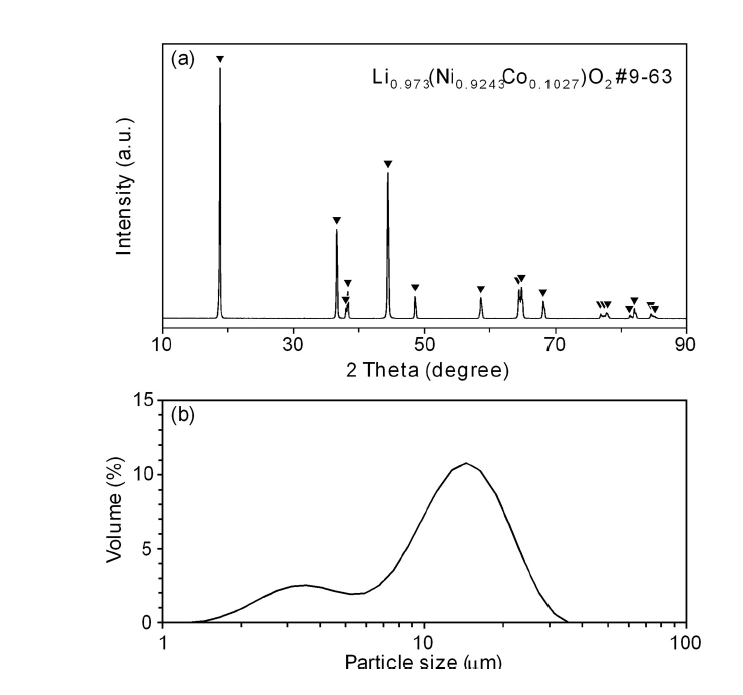
PDF - As the demand for lithium-ion batteries for electric vehicles is increasing, it is important to recover valuable metals from waste lithium-ion batteries. In this study, the effects of gas flow rate and hydrogen partial pressure on hydrogen reduction of NCM-based lithium-ion battery cathode materials were investigated. As the gas flow rate and hydrogen partial pressure increased, the weight loss rate increased significantly from the beginning of the reaction due to the reduction of NiO and CoO by hydrogen. At 700 °C and hydrogen partial pressure above 0.5 atm, Ni and Li2O were produced by hydrogen reduction. From the reduction product and Li recovery rate, the hydrogen reduction of NCM-based cathode materials was significantly affected by hydrogen partial pressure. The Li compounds recovered from the solution after water leaching of the reduction products were LiOH, LiOH·H2O, and Li2CO3, with about 0.02 wt% Al as an impurity.
-
Citations
Citations to this article as recorded by- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef
- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
- [Korean]
- Effect of Acid Leaching Conditions on the Properties of Cr Powder Produced by Self-propagating High-temperature Synthesis
- YongKwan Lee, YeongWoo Cho, ShinYoung Choi, SungGue Heo, Ju Won, KyoungTae Park, MiHye Lee, JaeJin Sim
- J Powder Mater. 2023;30(3):233-241. Published online June 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.3.233
- 696 View
- 3 Download
-
 Abstract
Abstract
 PDF
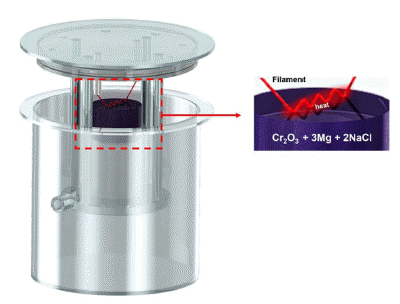
PDF In this study, we evaluated the effects of acid leaching on the properties of Cr powder synthesized using self-propagating high-temperature synthesis (SHS). Cr powder was synthesized from a mixture of Cr2O3 and magnesium (Mg) powders using the SHS Process, and the byproducts after the reaction were removed using acid leaching. The properties of the recovered Cr powder were analyzed via X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), particle size analysis (PSA), and oxygen content analysis. The results show that perfect selective leaching of Cr is challenging because of various factors such as incomplete reaction, reaction kinetics, the presence of impurities, and incompatibility between the acid and metal mixture. Therefore, this study provides essential information on the properties under acidic conditions during the production of high-quality Cr powder using a self-propagating high-temperature synthesis method.
- [Korean]
- Cobalt Recovery by Oxalic Acid and Hydroxide Precipitation from Waste Cemented Carbide Scrap Cobalt Leaching Solution
- Jaesung Lee, Mingoo Kim, Seulgi Kim, Dongju Lee
- J Korean Powder Metall Inst. 2021;28(6):497-501. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.497
- 1,153 View
- 15 Download
-
 Abstract
Abstract
 PDF
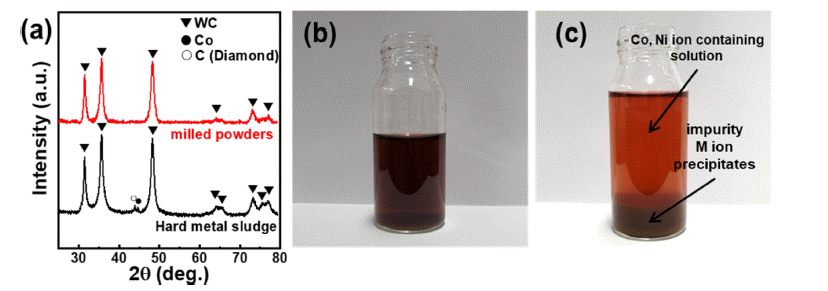
PDF Cobalt (Co) is mainly used to prepare cathode materials for lithium-ion batteries (LIBs) and binder metals for WC-Co hard metals. Developing an effective method for recovering Co from WC-Co waste sludge is of immense significance. In this study, Co is extracted from waste cemented carbide soft scrap via mechanochemical milling. The leaching ratio of Co reaches approximately 93%, and the leached solution, from which impurities except nickel are removed by pH titration, exhibits a purity of approximately 97%. The titrated aqueous Co salts are precipitated using oxalic acid and hydroxide precipitation, and the effects of the precipitating agent (oxalic acid and hydroxide) on the cobalt microstructure are investigated. It is confirmed that the type of Co compound and the crystal growth direction change according to the precipitation method, both of which affect the microstructure of the cobalt powders. This novel mechanochemical process is of significant importance for the recovery of Co from waste WC-Co hard metal. The recycled Co can be applied as a cemented carbide binder or a cathode material for lithium secondary batteries.
- [English]
- A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
- Jei-Pil Wang, Jae-Jung Pyo, Se-Ho Ahn, Dong-Hyeon Choi, Byeong-Woo Lee, Dong-Won Lee
- J Korean Powder Metall Inst. 2018;25(4):296-301. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.296
- 3,170 View
- 73 Download
- 11 Citations
-
 Abstract
Abstract
 PDF
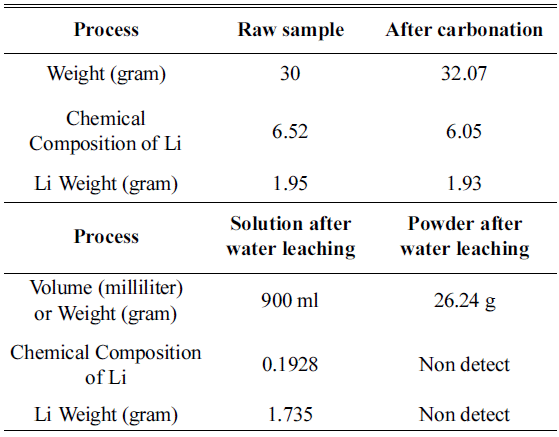
PDF In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered.
-
Citations
Citations to this article as recorded by- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
Amir Hossein Mohammad Zadeh, Spencer Cunnigham, Devon Gray, Sevan Bedrossian, Baian Almusned, Jeffrey Daniel Henderson, Gisele Azimi
Waste Management.2026; 212: 115339. CrossRef - Hydrogen Reduction of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries
Jae-Ho Hwang, Sang-Yeop Lee, So-Yeong Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(3): 34. CrossRef - Reduction Roasting of Cathode Materials of NCM Based Lithium-ion Batteries Using CH4(g)
Jae-Ho Hwang, Sang-Yeop Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(4): 48. CrossRef - High‐Rate Rechargeable Li/SOCl2 Batteries Enabled by Cobalt Phthalocyanine Cathodic Catalysts
Yingxuan Song, Haibo Ouyang, Zhanwei Xu, Zeyang Zhang, Kang Li, Jianfeng Huang, Zhi Li, Tian Wang, Jun Yang
Small.2025;[Epub] CrossRef - Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef - Metals Recovery from Spent Lithium-ion Batteries Cathode Via Hydrogen Reduction-water Leaching-carbothermic or Hydrogen Reduction Process
Tahereh Rostami, Behnam Khoshandam
Mining, Metallurgy & Exploration.2024; 41(3): 1485. CrossRef - Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(5): 923. CrossRef - Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(12): 2000. CrossRef - Environmentally Friendly Recovery of Lithium from Lithium–Sulfur Batteries
Lilian Schwich, Bernd Friedrich
Metals.2022; 12(7): 1108. CrossRef - Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Lilian Schwich, Tom Schubert, Bernd Friedrich
Metals.2021; 11(2): 177. CrossRef - Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem
Jiadong Yu, Quanyin Tan, Jinhui Li
Journal of Cleaner Production.2020; 255: 120269. CrossRef
- Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 821 View
- 4 Download
-
 Abstract
Abstract
 PDF
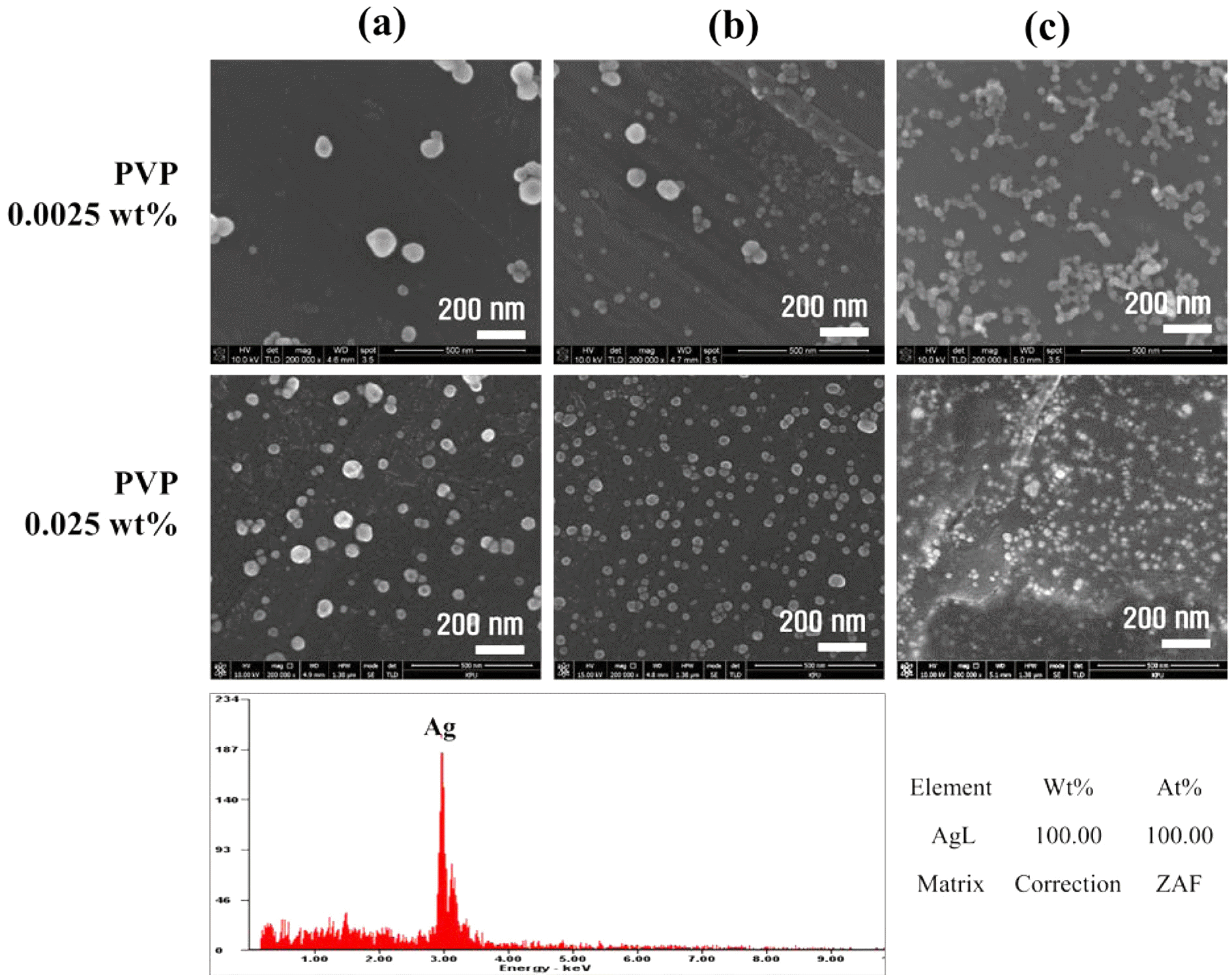
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- Recovery of Tungsten from WC/Co Hardmetal Sludge by Alkaline Leaching Hydrometallurgy Process
- Gil-Geun Lee, Ji-Eun Kwon
- J Korean Powder Metall Inst. 2016;23(5):372-378. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.372
- 705 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF This study focuses on the development of an alkaline leaching hydrometallurgy process for the recovery of tungsten from WC/Co hardmetal sludge, and an examination of the effect of the process parameters on tungsten recovery. The alkaline leaching hydrometallurgy process has four stages, i.e., oxidation of the sludge, leaching of tungsten by NaOH, refinement of the leaching solution, and precipitation of tungsten. The WC/Co hardmetal sludge oxide consists of WO3 and CoWO4. The leaching of tungsten is most affected by the leaching temperature, followed by the NaOH concentration and the leaching time. About 99% of tungsten in the WC/Co hardmetal sludge is leached at temperatures above 90°C and a NaOH concentration above 15%. For refinement of the leaching solution, pH control of the solution using HCl is more effective than the addition of Na2S·9H2O. The tungsten is precipitated as high-purity H2WO4·H2O by pH control using HCl. With decreasing pH of the solution, the tungsten recovery rate increases and then decrease. About 93% of tungsten in the WC/Co hardmetal sludge is recovered by the alkaline leaching hydrometallurgy process.
-
Citations
Citations to this article as recorded by- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
Min Soo Park, Jong-Min Gwak, Kyeong-mi Jang, Gook-Hyun Ha
Powder Metallurgy.2023; 66(5): 688. CrossRef
- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
- [Korean]
- Leaching behavior of Ga and In from MOCVD dust
- Kyung-Soo Park, Basudev Swain, Lee Seung Kang, Chan Gi Lee, Hyun Seon Hong, Jong-Gil Shim, Jeung-Jin Park
- J Korean Powder Metall Inst. 2014;21(3):202-206. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.202
- 875 View
- 5 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Leaching of MOCVD dust in the LED industry is an essential stage for hydro-metallurgical recovery of pure Ga and In. To recover Ga and In, the leaching behavior of MOCVD scrap of an LED, which contains significant amounts of Ga, In, Al and Fe in various phases, has been investigated. The leaching process must be performed effectively to maximize recovery of Ga and In metals using the most efficient lixiviant. Crystalline structure and metallic composition of the raw MOCVD dust were analyzed prior to digestion. Subsequently, various mineral acids were tested to comprehensively study and optimize the leaching parameters such as acidity, pulp density, temperature and time. The most effective leaching of Ga and In was observed for a boiling 4 M HCl solution vigorously stirred at 400 rpm. Phase transformation of GaN into gallium oxide by heat treatment also improved the leaching efficiency of Ga. Subsequently high purity Ga and In can be recovered by series of hydro processes.
-
Citations
Citations to this article as recorded by- Selective Solvent Extraction of In from Synthesis Solution of MOCVD Dust using D2EHPA
Byoungyong Im, Basudev Swain, Chan Gi Lee, Jae Layng Park, Kyung-Soo Park, Jong-Gil Shim, Jeung-Jin Park
Journal of the Korean Institute of Resources Recycling.2015; 24(5): 80. CrossRef - Fabrication of High Purity Ga-containing Solution using MOCVD dust
Duk-Hee Lee, Jin-Ho Yoon, Kyung-Soo Park, Myung-Hwan Hong, Chan-Gi Lee, Jeung-Jin Park
Journal of the Korean Institute of Resources Recycling.2015; 24(4): 50. CrossRef - Influence of Oxidation Temperatures on the Structure and the Microstructure of GaN MOCVD Scraps
Hyun Seon Hong, Joong Woo Ahn
journal of Korean Powder Metallurgy Institute.1970; 22(4): 278. CrossRef
- Selective Solvent Extraction of In from Synthesis Solution of MOCVD Dust using D2EHPA
TOP
 KPMI
KPMI


 First
First Prev
Prev


