Search
- Page Path
- HOME > Search
- [English]
- SnF2-Induced LiF Interphase for Stable Lithium Metal Anodes with Suppressed Dendrite Growth
- Yeong Hoon Jeon, Seul Ki Choi, Yun Seung Nah, Wonil Shin, Yong-Ho Choa, Minho Yang
- J Powder Mater. 2025;32(3):212-221. Published online June 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00164

- 1,738 View
- 53 Download
-
 Abstract
Abstract
 PDF
PDF - Lithium (Li) metal is a promising anode for next-generation batteries due to its high capacity, low redox potential, and low density. However, dendrite growth and interfacial instability limit its use. In this study, an artificial solid electrolyte interphase layer of LiF and Li-Sn (LiF@Li-Sn) was fabricated by spray-coating SnF2 onto Li. The LiF@Li-Sn anode exhibited improved air stability and electrochemical performance. Electrochemical impedance spectroscopy indicated a charge transfer resistance of 25.2 Ω after the first cycle. In symmetric cells, it maintained a low overpotential of 27 mV after 250 cycles at 2 mA/cm2, outperforming bare Li. In situ microscopy confirmed dendrite suppression during plating. Full cells with NMC622 cathodes and LiF@Li-Sn anodes delivered 130.8 mAh/g with 79.4% retention after 300 cycles at 1 C and 98.8% coulombic efficiency. This coating effectively stabilized the interface and suppressed dendrites, with promising implications for practical lithium metal batteries.
- [English]
- A Review of Inorganic Solid Electrolytes for All-Solid-State Lithium Batteries: Challenges and Progress
- Seul Ki Choi, Jaehun Han, Gi Jeong Kim, Yeon Hee Kim, Jaewon Choi, MinHo Yang
- J Powder Mater. 2024;31(4):293-301. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00206
- 11,674 View
- 282 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
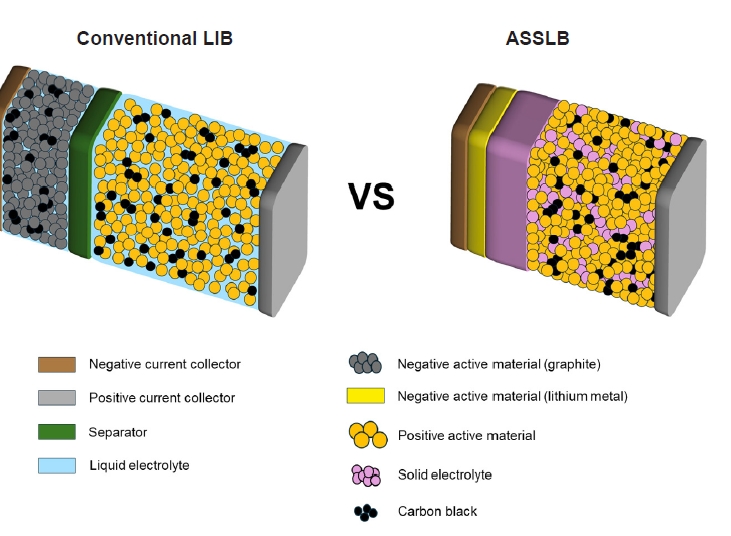
PDF - All-solid-state lithium batteries (ASSLBs) are receiving attention as a prospective next-generation secondary battery technology that can reduce the risk of commercial lithium-ion batteries by replacing flammable organic liquid electrolytes with non-flammable solid electrolytes. The practical application of ASSLBs requires developing robust solid electrolytes that possess ionic conductivity at room temperature on a par with that of organic liquids. These solid electrolytes must also be thermally and chemically stable, as well as compatible with electrode materials. Inorganic solid electrolytes, including oxide and sulfide-based compounds, are being studied as promising future candidates for ASSLBs due to their higher ionic conductivity and thermal stability than polymer electrolytes. Here, we present the challenges currently facing the development of oxide and sulfide-based solid electrolytes, as well as the research efforts underway aiming to resolve these challenges.
-
Citations
Citations to this article as recorded by- A facile synthesis of bulk LiPON in solution for solid-state electrolytes
Osma J. Gomez, Adam Antar, Alex T. Hall, Leopoldo Tapia-Aracayo, Joshua Seo, Nam Kim, Zihan Sun, Ryan Lim, Fu Chen, Yue Li, John Cumings, Gary Rubloff, Sang Bok Lee, David Stewart, Yang Wang
Journal of Materials Chemistry A.2025; 13(34): 28368. CrossRef - Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef - Garnet-type LLZO electrolytes for solid-state lithium batteries: Interfaces, conductivity, in-situ processing, and industrial prospects
Kaleab Habtamu Ayalew, Nithyadharseni Palaniyandy, Mkhulu K. Mathe, Phumlani F. Msomi
Chemical Engineering Journal.2025; 524: 168098. CrossRef
- A facile synthesis of bulk LiPON in solution for solid-state electrolytes
- [Korean]
- Effect of Chelating Agent on Li1.5Al0.5Ti1.5(PO4)3 Particles by Sol-gel Method and Densification
- SungJoon Ryu, Seul Ki Choi, Jong Ho Won, MinHo Yang
- J Powder Mater. 2023;30(5):394-401. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.394
- 1,762 View
- 48 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Li1.5Al0.5Ti1.5(PO4)3 (LATP) is considered to be one of the promising solid-state electrolytes owing to its excellent chemical and thermal stability, wide potential range (~5.0 V), and high ionic conductivity (~10-4 S/cm). LATP powders are typically prepared via the sol-gel method by adding and mixing nitrate or alkoxide precursors with chelating agents. Here, the thermal properties, crystallinity, density, particle size, and distribution of LATP powders based on chelating agents (citric acid, acetylacetone, EDTA) are compared to find the optimal conditions for densely sintered LATP with high purity. In addition, the three types of LATP powders are utilized to prepare sintered solid electrolytes and observe the microstructure changes during the sintering process. The pyrolysis onset temperature and crystallization temperature of the powder samples are in the order AC-LATP > CA-LATP > ED-LATP, and the LATP powder utilizing citric acid exhibits the highest purity, as no secondary phase other than LiTi2PO4 phase is observed. LATP with citric acid and acetylacetone has a value close to the theoretical density (2.8 g/cm3) after sintering. In comparison, LATP with EDTA has a low sintered density (2.2 g/cm3) because of the generation of many pores after sintering.
-
Citations
Citations to this article as recorded by- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
Seo Yun Jung, Jaehun Han, Seul Ki Choi, Se Youn Cho, Jong Ho Won, Jaewon Choi, Minho Yang
Chemical Engineering Journal.2025; 522: 167302. CrossRef
- Uniform lithium deposition using Cu teepee structures for anode-free lithium metal batteries
- [Korean]
- A Study on the Microstructures and Ionic Conductivity of Li1.3Al0.3Ti1.7(PO4)3 with Different Synthesis Routes
- Seul Ki Choi, Jeawon Choi, MinHo Yang
- J Powder Mater. 2023;30(2):107-115. Published online April 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.2.107
- 2,125 View
- 48 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
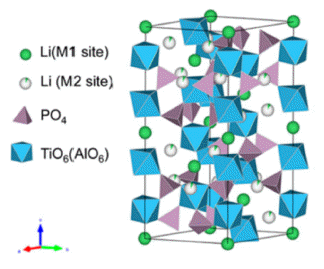
PDF Li1.3Al0.3Ti1.7(PO4)3(LATP) is considered a promising material for all-solid-state lithium batteries owing to its high moisture stability, wide potential window (~6 V), and relatively high ion conductivity (10-3–10-4 S/cm). Solid electrolytes based on LATP are manufactured via sintering, using LATP powder as the starting material. The properties of the starting materials depend on the synthesis conditions, which affect the microstructure and ionic conductivity of the solid electrolytes. In this study, we synthesize the LATP powder using sol-gel and co-precipitation methods and characterize the physical properties of powder, such as size, shape, and crystallinity. In addition, we have prepared a disc-shaped LATP solid electrolyte using LATP powder as the starting material. In addition, X-ray diffraction, scanning electron microscopy, and electrochemical impedance spectroscopic measurements are conducted to analyze the grain size, microstructures, and ion conduction properties. These results indicate that the synthesis conditions of the powder are a crucial factor in creating microstructures and affecting the conduction properties of lithium ions in solid electrolytes.
-
Citations
Citations to this article as recorded by- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
Gwanhee Song, Bojoong Kim, Inkook Hwang, Jiwon Kim, Jinmo Kim, Chang-Bun Yoon
Materials.2025; 18(3): 609. CrossRef
- Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
- [Korean]
- The Synthesis of Lithium Lanthanum Titanium Oxide for Solid Electrolyte via Ultrasonic Spray Pyrolysis
- Jaeseok Roh, MinHo Yang, Kun-Jae Lee
- J Powder Mater. 2022;29(6):485-491. Published online December 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.6.485

- 1,455 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF Lithium lanthanum titanium oxide (LLTO) is a promising ceramic electrolyte because of its high ionic conductivity at room temperature, low electrical conductivity, and outstanding physical properties. Several routes for the synthesis of bulk LLTO are known, in particular, solid-state synthesis and sol-gel method. However, the extremely low ionic conductivity of LLTO at grain boundaries is one of the major problems for practical applications. To diminish the grain boundary effect, the structure of LLTO is tuned to nanoscale morphology with structures of different dimensionalities (0D spheres, and 1D tubes and wires); this strategy has great potential to enhance the ion conduction by intensifying Li diffusion and minimizing the grain boundary resistance. Therefore, in this work, 0D spherical LLTO is synthesized using ultrasonic spray pyrolysis (USP). The USP method primarily yields spherical particles from the droplets generated by ultrasonic waves passed through several heating zones. LLTO is synthesized using USP, and the effects of each precursor and their mechanisms as well as synthesis parameters are analyzed and discussed to optimize the synthesis. The phase structure of the obtained materials is analyzed using X-ray diffraction, and their morphology and particle size are analyzed using field-emission scanning electron microscopy.
- [English]
- Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
- Bong Gill Choi, MinHo Yang
- J Korean Powder Metall Inst. 2018;25(3):203-207. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2017.25.3.203
- 618 View
- 3 Download
-
 Abstract
Abstract
 PDF
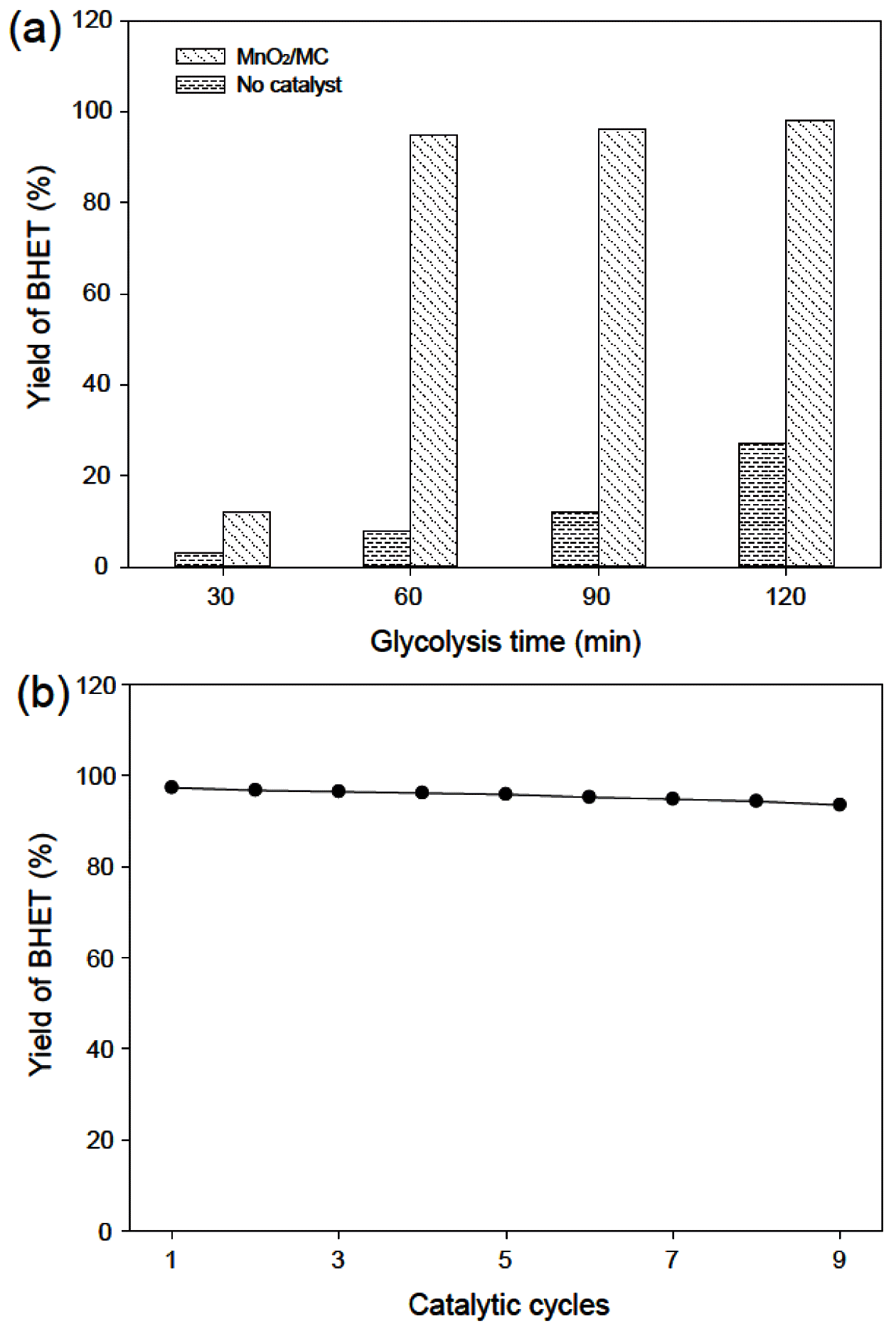
PDF Plastic pollution is threatening human health and ecosystems, resulting in one of the biggest challenges that humanity has ever faced. Therefore, this study focuses on the preparation of macroporous carbon from biowaste (MC)-supported manganese oxide (MnO2) as an efficient, reusable, and robust catalyst for the recycling of poly(ethylene terephthalate) (PET) waste. As-prepared MnO2/MC composites have a hierarchical pore network and a large surface area (376.16 m2/g) with a narrow size distribution. MnO2/MC shows a maximum yield (98%) of bis(2-hydroxyethyl)terephthalate (BHET) after glycolysis reaction for 120 min. Furthermore, MnO2/MC can be reused at least nine times with a negligible decrease in BHET yield. Based on this remarkable catalytic performance, we expect that MnO2-based heterogeneous catalysts have the potential to be introduced into the PET recycling industry.
TOP
 KPMI
KPMI


 First
First Prev
Prev


