Search
- Page Path
- HOME > Search
- [English]
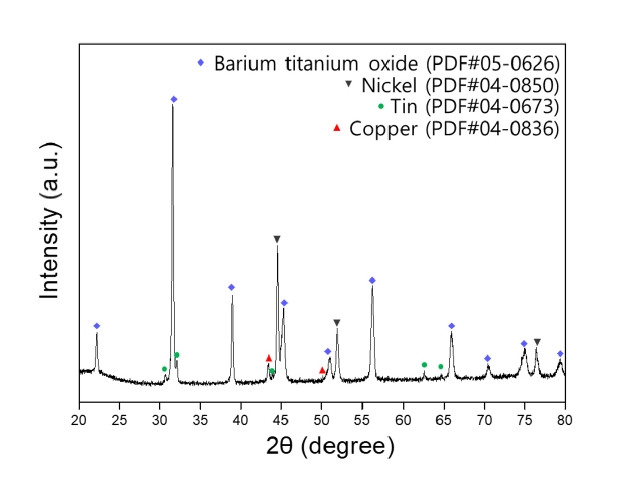
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- Haein Shin, Kun-Jae Lee
- J Powder Mater. 2024;31(5):374-381. Published online October 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00192
- 2,236 View
- 55 Download
-
 Abstract
Abstract
 PDF
PDF - The development of the electronics industry has led to an increased demand for the manufacture of MLCC (Multilayer Ceramic Capacitors), which in turn is expected to result in a rise in MLCC waste. The MLCC contains various metals, notably barium, titanium, and nickel, whose disposal is anticipated to increase correspondingly. Recently, recycling technologies for electronic waste have garnered attention as they address waste management and raw material supply challenges. This paper investigates the recovery of barium, nickel, and titanium from the MLCC by a hydrometallurgical process. Using citric acid, which is an organic acid, the metal inside the MLCC was leached. Additionally, metal materials were recovered through precipitation and complexing processes. As a result, barium and titanium were recovered from the leachate of the waste MLCC, and 93% of the nickel-based powder was recovered. Furthermore, the optimal recovery process conditions for recycling these metal elements were investigated.
- [Korean]
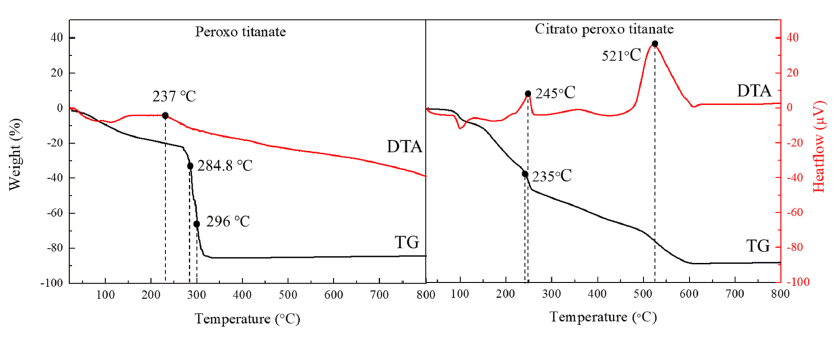
- A Study on the Preparation and Growth Mechanism of Titanium Dioxide using Organic-Inorganic Hybrid Titanium Complex
- Yubin Kang, Jin-Ju Choi, Nam Hun Kwon, Dae-Guen Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(6):487-492. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.487
- 1,151 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Titanium dioxide (TiO2) is a typical inorganic material that has an excellent photocatalytic property and a high refractive index. It is used in water/air purifiers, solar cells, white pigments, refractory materials, semiconductors, etc.; its demand is continuously increasing. In this study, anatase and rutile phase titanium dioxide is prepared using hydroxyl and carboxyl; the titanium complex and its mechanism are investigated. As a result of analyzing the phase transition characteristics by a heat treatment temperature using a titanium complex having a hydroxyl group and a carboxyl group, it is confirmed that the material properties were different from each other and that the anatase and rutile phase contents can be controlled. The titanium complexes prepared in this study show different characteristics from the titania-formation temperatures of the known anatase and rutile phases. It is inferred that this is due to the change of electrostatic adsorption behavior due to the complexing function of the oxygen sharing point, which crystals of the TiO6 structure share.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
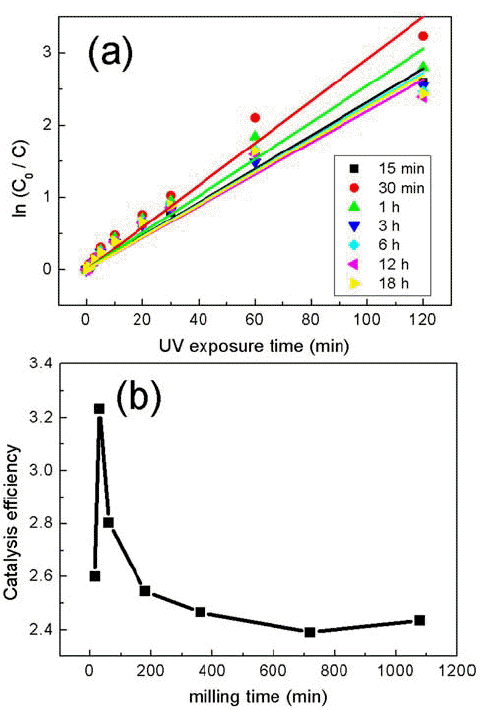
- Photocatalysis of TiO2/WO3 Composites Synthesized by Ball Milling
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2018;25(4):316-321. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.316
- 779 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF Composites of P25 TiO2 and hexagonal WO3 nanorods are synthesized through ball-milling in order to study photocatalytic properties. Various composites of TiO2/WO3 are prepared by controlling the weight percentages (wt%) of WO3, in the range of 1–30 wt%, and milling time to investigate the effects of the composition ratio on the photocatalytic properties. Scanning electron microscopy, x-ray diffraction, and transmission electron microscopy are performed to characterize the structure, shape and size of the synthesized composites of TiO2/WO3. Methylene blue is used as a test dye to analyze the photocatalytic properties of the synthesized composite material. The photocatalytic activity shows that the decomposition efficiency of the dye due to the photocatalytic effect is the highest in the TiO2/WO3 (3 wt%) composite, and the catalytic efficiency decreases sharply when the amount of WO3 is further increased. As the amount of WO3 added increases, dye-removal by adsorption occurs during centrifugation, instead of the decomposition of dyes by photocatalysts. Finally, TiO2/WO3 (3 wt%) composites are synthesized with various milling times. Experimental results show that the milling time has the best catalytic efficiency at 30 min, after which it gradually decreases. There is no significant change after 1 hour.
- [Korean]
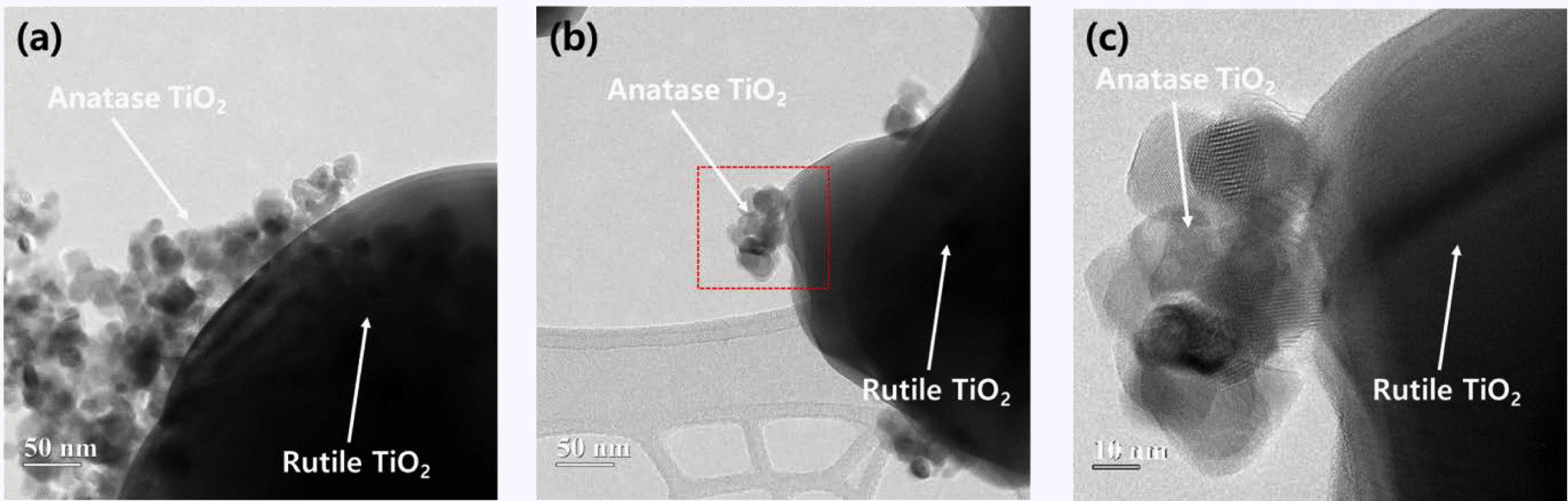
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
- Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
- J Korean Powder Metall Inst. 2018;25(3):257-262. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.257
- 1,136 View
- 9 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF The coupling of two semiconducting materials is an efficient method to improve photocatalytic activity via the suppression of recombination of electron-hole pairs. In particular, the coupling between two different phases of TiO2, i.e., anatase and rutile, is particularly attractive for photocatalytic activity improvement of rutile TiO2 because these coupled TiO2 powders can retain the benefits of TiO2, one of the best photocatalysts. In this study, anatase TiO2 nanoparticles are synthesized and coupled on the surface of rutile TiO2 powders using a microemulsion method and heat treatment. Triton X-100, as a surfactant, is used to suppress the aggregation of anatase TiO2 nanoparticles and disperse anatase TiO2 nanoparticles uniformly on the surface of rutile TiO2 powders. Rutile TiO2 powders coupled with anatase TiO2 nanoparticles are successfully prepared. Additionally, we compare the photocatalytic activity of these rutile-anatase coupled TiO2 powders under ultraviolet (UV) light and demonstrate that the reason for the improvement of photocatalytic activity is microstructural.
-
Citations
Citations to this article as recorded by- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Min-Sang Kim, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, Se-Hoon Kim
Catalysts.2021; 11(8): 987. CrossRef - Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 395. CrossRef
- Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
- [Korean]
- Synthesis of Pt@TiO2 Nano-composite via Photochemical Reduction Method
- Ji Young Kim, Jong Min Byun, Jin Woo Kim, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(2):119-123. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.119
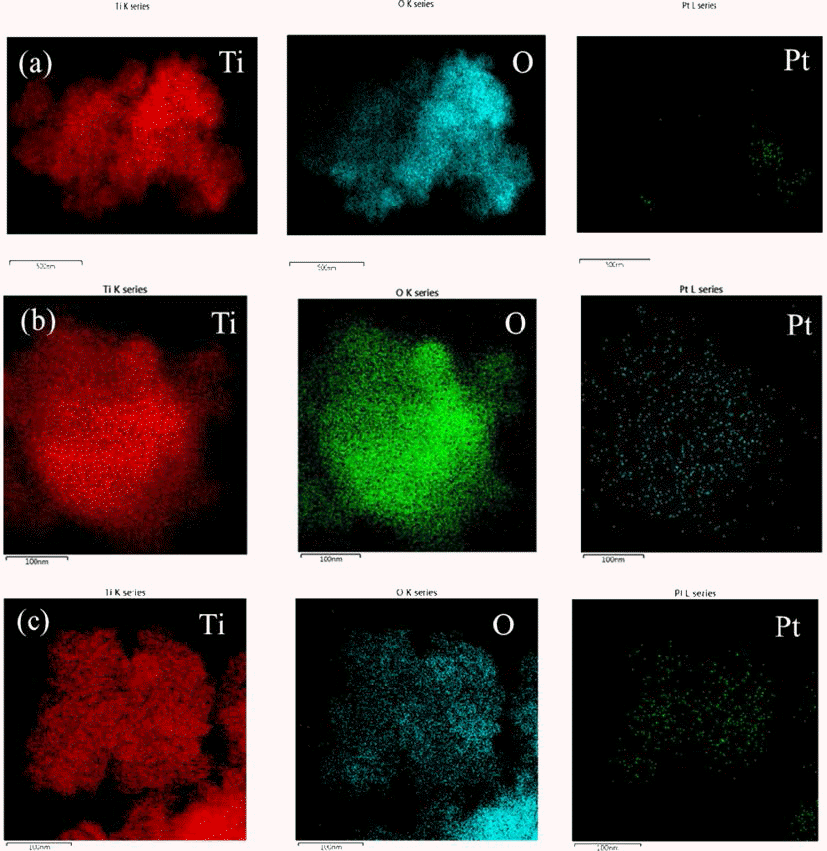
- 827 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Pt has been widely used as catalyst for fuel cell and exhausted gas clean systems due to its high catalytic activity. Recently, there have been researches on fabricating composite materials of Pt as a method of reducing the amount of Pt due to its high price. One of the approaches for saving Pt used as catalyst is a core shell structure consisting of Pt layer on the core of the non-noble metal. In this study, the synthesis of Pt shell was conducted on the surface of TiO2 particle, a non-noble material, by applying ultraviolet (UV) irradiation. Anatase TiO2 particles with the average size of 20~30 nm were immersed in the ethanol dissolved with Pt precursor of H2PtCl6∙6H2O and exposed to UV irradiation with the wavelength of 365 nm. It was confirmed that Pt nano-particles were formed on the surface of TiO2 particles by photochemical reduction of Pt ion from the solution. The morphology of the synthesized Pt@TiO2 nano-composite was examined by TEM (Transmission Electron Microscopy).
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
Hyeong-Chul Kim, Jae-Kil Han
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 33. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


