Search
- Page Path
- HOME > Search
- [English]
- SnF2-Induced LiF Interphase for Stable Lithium Metal Anodes with Suppressed Dendrite Growth
- Yeong Hoon Jeon, Seul Ki Choi, Yun Seung Nah, Wonil Shin, Yong-Ho Choa, Minho Yang
- J Powder Mater. 2025;32(3):212-221. Published online June 30, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00164

- 1,692 View
- 50 Download
-
 Abstract
Abstract
 PDF
PDF - Lithium (Li) metal is a promising anode for next-generation batteries due to its high capacity, low redox potential, and low density. However, dendrite growth and interfacial instability limit its use. In this study, an artificial solid electrolyte interphase layer of LiF and Li-Sn (LiF@Li-Sn) was fabricated by spray-coating SnF2 onto Li. The LiF@Li-Sn anode exhibited improved air stability and electrochemical performance. Electrochemical impedance spectroscopy indicated a charge transfer resistance of 25.2 Ω after the first cycle. In symmetric cells, it maintained a low overpotential of 27 mV after 250 cycles at 2 mA/cm2, outperforming bare Li. In situ microscopy confirmed dendrite suppression during plating. Full cells with NMC622 cathodes and LiF@Li-Sn anodes delivered 130.8 mAh/g with 79.4% retention after 300 cycles at 1 C and 98.8% coulombic efficiency. This coating effectively stabilized the interface and suppressed dendrites, with promising implications for practical lithium metal batteries.
- [Korean]
- Synthesis of Carbon Coated Nickel Cobalt Sulfide Yolk-shell Microsphere and Their Application as Anode Materials for Sodium Ion Batteries
- Hyo Yeong Seo, Gi Dae Park
- J Powder Mater. 2023;30(5):387-393. Published online October 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.5.387
- 737 View
- 15 Download
-
 Abstract
Abstract
 PDF
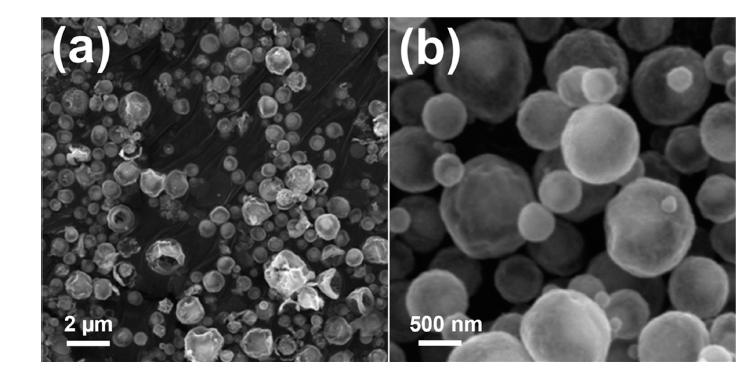
PDF Transition metal chalcogenides are promising cathode materials for next-generation battery systems, particularly sodium-ion batteries. Ni3Co6S8-pitch-derived carbon composite microspheres with a yolk-shell structure (Ni3Co6S8@C-YS) were synthesized through a three-step process: spray pyrolysis, pitch coating, and post-heat treatment process. Ni3Co6S8@C-YS exhibited an impressive reversible capacity of 525.2 mA h g-1 at a current density of 0.5 A g-1 over 50 cycles when employed as an anode material for sodium-ion batteries. However, Ni3Co6S8 yolk shell nanopowder (Ni3Co6S8-YS) without pitch-derived carbon demonstrated a continuous decrease in capacity during charging and discharging. The superior sodium-ion storage properties of Ni3Co6S8@C-YS were attributed to the pitchderived carbon, which effectively adjusted the size and distribution of nanocrystals. The carbon-coated yolk-shell microspheres proposed here hold potential for various metal chalcogenide compounds and can be applied to various fields, including the energy storage field.
- [Korean]
- Electrochemical Properties of Ball-milled Tin-Graphite Composite Anode Materials for Lithium-Ion Battery
- Tae-Hui Lee, Hyeon-A Hong, Kwon-Koo Cho, Yoo-Young Kim
- J Korean Powder Metall Inst. 2021;28(6):462-469. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.462

- 1,169 View
- 10 Download
-
 Abstract
Abstract
 PDF
PDF Tin/graphite composites are prepared as anode materials for Li-ion batteries using a dry ball-milling process. The main experimental variables in this work are the ball milling time (0–8 h) and composition ratio (tin:graphite=5:95, 15:85, and 30:70 w/w) of graphite and tin powder. For comparison, a tin/graphite composite is prepared using wet ball milling. The morphology and structure of the different tin/graphite composites are investigated using X-ray diffraction, Raman spectroscopy, energy-dispersive X-ray spectroscopy, and scanning and transmission electron microscopy. The electrochemical properties of the samples are also examined. The optimal dry ball milling time for the uniform mixing of graphite and tin is 6 h in a graphite-30wt.%Sn sample. The electrode prepared from the composite that is dry-ballmilled for 6 h exhibits the best cycle performance (discharge capacity after 50th cycle: 308 mAh/g and capacity retention: 46%). The discharge capacity after the 50th cycle is approximately 112 mAh/g, higher than that when the electrode is composed of only graphite (196 mAh/g after 50th cycle). This result indicates that it is possible to manufacture a tin/graphite composite anode material that can effectively buffer the volume change that occurs during cycling, even using a simple dry ball-milling process.
- [Korean]
- Synthesis of the Multi-layered SnO Nanoparticles and Enhanced Performance of Lithium-Ion Batteries by Heat treatment
- So Yi Lee, Yoon Myung, Kyu-Tae Lee, Jaewon Choi
- J Korean Powder Metall Inst. 2021;28(6):455-461. Published online December 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.6.455

- 1,675 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, multilayered SnO nanoparticles are prepared using oleylamine as a surfactant at 165°C. The physical and chemical properties of the multilayered SnO nanoparticles are determined by transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). Interestingly, when the multilayered SnO nanoparticles are heated at 400°C under argon for 2 h, they become more efficient anode materials, maintaining their morphology. Heat treatment of the multilayered SnO nanoparticles results in enhanced discharge capacities of up to 584 mAh/g in 70 cycles and cycle stability. These materials exhibit better coulombic efficiencies. Therefore, we believe that the heat treatment of multilayered SnO nanoparticles is a suitable approach to enable their application as anode materials for lithium-ion batteries.
-
Citations
Citations to this article as recorded by- Synthesis and electrochemical properties of multi-layered SnO/rGO composite as anode materials for sodium ion batteries
So Yi Lee, Honggyu Seong, Geongil Kim, Youngho Jin, Joon Ha Moon, Wonbin Nam, Sung Kuk Kim, MinHo Yang, Jaewon Choi
Applied Surface Science.2023; 612: 155859. CrossRef
- Synthesis and electrochemical properties of multi-layered SnO/rGO composite as anode materials for sodium ion batteries
- [Korean]
- Fabrication of Fe3O4/Fe/Graphene nanocomposite powder by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ji-Seub Choi, Hoi-Jin Lee, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2017;24(4):308-314. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.308
- 972 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
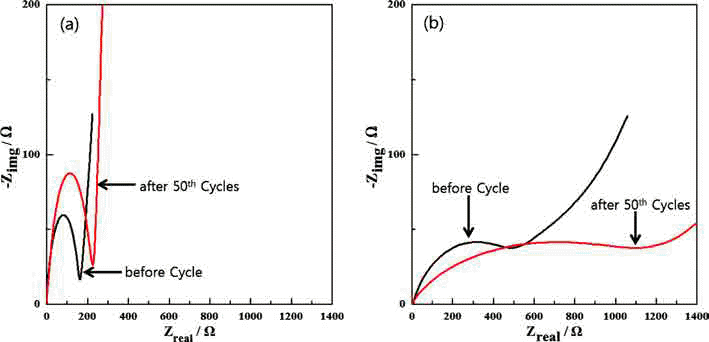
PDF Fe3O4/Fe/graphene nanocomposite powder is synthesized by electrical wire explosion of Fe wire and dispersed graphene in deionized water at room temperature. The structural and electrochemical characteristics of the powder are characterized by the field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, field-emission transmission electron microscopy, cyclic voltammetry, and galvanometric discharge-charge method. For comparison, Fe3O4/Fe nanocomposites are fabricated under the same conditions. The Fe3O4/Fe nanocomposite particles, around 15-30 nm in size, are highly encapsulated in a graphene matrix. The Fe3O4/Fe/graphene nanocomposite powder exhibits a high initial charge specific capacity of 878 mA/g and a high capacity retention of 91% (798 mA/g) after 50 cycles. The good electrochemical performance of the Fe3O4/Fe/graphene nanocomposite powder is clearly established by comparison of the results with those obtained for Fe3O4/Fe nanocomposite powder and is attributed to alleviation of volume change, good distribution of electrode active materials, and improved electrical conductivity upon the addition of graphene.
-
Citations
Citations to this article as recorded by- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
Kyunbae Lee, Joonsik Lee, Byung Mun Jung, Byeongjin Park, Taehoon Kim, Sang Bok Lee
Applied Surface Science.2019; 478: 733. CrossRef
- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
- [Korean]
- Effects of Porous Microstructure on the Electrochemical Properties of Si-Ge-Al Base Anode Materials for Li-ion Rechargeable Batteries
- Chung Rae Cho, Myeong Geun Kim, Keun Yong Sohn, Won-Wook Park
- J Korean Powder Metall Inst. 2017;24(1):24-28. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.24
- 627 View
- 3 Download
-
 Abstract
Abstract
 PDF
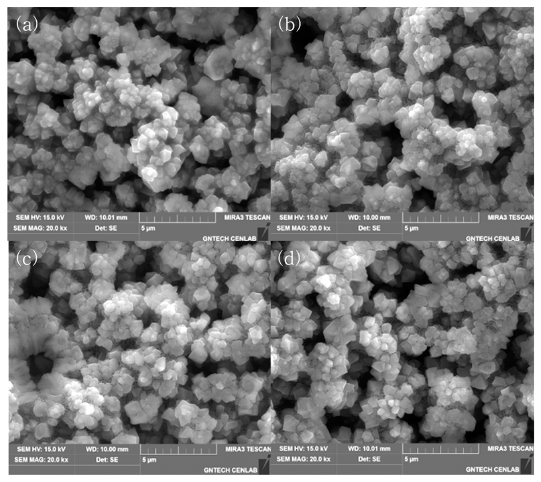
PDF Silicon alloys are considered promising anode active materials to replace Li-ion batteries by graphite powder, because they have a relatively high capacity of up to 4200 mAh/g, and are environmentally friendly and inexpensive ECO-materials. However, its poor charge/discharge properties, induced by cracking during cycles, constitute their most serious problem as anode electrode. In order to solve these problems, Si-Ge-Al alloys with porous structure are designed as anode alloy powders, to improve cycling stability. The alloys are melt-spun to obtain the rapidly solidified ribbons, and then ball-milled to make fine powders. The powders are etched using 1 M HCl solution, which gives the powders a porous structure by removing the element Al. Subsequently, in this study, the microstructures and the characteristics of the etched powders are evaluated for application as anode materials. As a result, the etched porous powder shows better electrochemical properties than as-milled Si-Ge-Al powder.
- [Korean]
- Fabrication of Carbon-coated Tin Nano-powders by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ju-Suck Song, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2016;23(4):317-324. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.317
- 946 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
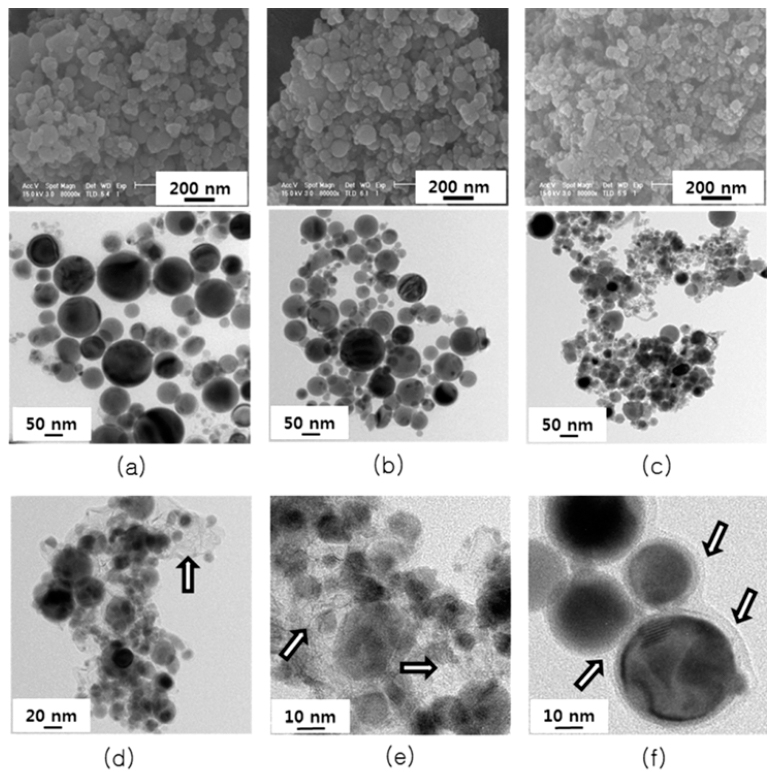
PDF Tin is one of the most promising anode materials for next-generation lithium-ion batteries with a high energy density. However, the commercialization of tin-based anodes is still hindered due to the large volume change (over 260%) upon lithiation/delithiation cycling. To solve the problem, many efforts have been focused on enhancing structural stability of tin particles in electrodes. In this work, we synthesize tin nano-powders with an amorphous carbon layer on the surface and surroundings of the powder by electrical wire explosion in alcohol-based liquid media at room temperature. The morphology and microstructures of the powders are characterized by scanning electron microscopy, Xray diffraction, Raman spectroscopy, and transmission electron microscopy. The electrochemical properties of the powder for use as an anode material for lithium-ion battery are evaluated by cyclic voltammetry and a galvanometric dischargecharge method. It is shown that the carbon-coated tin nano-powders prepared in hexanol media exhibit a high initial charge specific capacity of 902 mAh/g and a high capacity retention of 89% after 50 cycles.
-
Citations
Citations to this article as recorded by- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
Ji-Seub Choi, Yeon-Ju Lee, Hoi-Jin Lee, Gyu-Bong Cho, Jai-Won Byeon, Hyo-Jun Ahn, Ki-Won Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Journal of Alloys and Compounds.2020; 843: 155892. CrossRef - Fabrication of multilayer graphene-encapsulated Sn/SnO2 nanocomposite as an anode material for lithium-ion batteries and its electrochemical properties
Ju-Seok Song, Gyu-Bong Cho, Ki-Won Kim, Hyo-Jun Ahn, Hye-Sung Kim, Jou-Hyeon Ahn, Kwon-Koo Cho
Applied Surface Science.2019; 481: 736. CrossRef
- Optimization of carbon coating thickness to prevent crack generation in Sn nanoparticles during charge/discharge process and their electrochemical properties
- [English]
- Preparation and Characterization of Porous Silicon and Carbon Composite as an Anode Material for Lithium Rechargeable Batteries
- Junsoo Park, Jae-won Lee
- J Korean Powder Metall Inst. 2015;22(1):15-20. Published online February 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.1.15
- 730 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The composite of porous silicon (Si) and amorphous carbon (C) is prepared by pyrolysis of a nano-porous Si + pitch mixture. The nano-porous Si is prepared by mechanical milling of magnesium powder with silicon monoxide (SiO) followed by removal of MgO with hydrochloric acid (etching process). The Brunauer-Emmett-Teller (BET) surface area of porous Si (64.52 m2g−1) is much higher than that before etching Si/MgO (4.28 m2g−1) which indicates pores are formed in Si after the etching process. Cycling stability is examined for the nano-porous Si + C composite and the result is compared with the composite of nonporous Si + C. The capacity retention of the former composite is 59.6% after 50 charge/discharge cycles while the latter shows only 28.0%. The pores of Si formed after the etching process is believed to accommodate large volumetric change of Si during charging and discharging process.
- [Korean]
- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
- Sung-Soo Ryu, Hui-Sik Kim, Seongwon Kim, Hyung-Tae Kim, Hae-Won Cheong, Sung-Min Lee
- J Korean Powder Metall Inst. 2014;21(5):331-337. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.331
- 698 View
- 9 Download
-
 Abstract
Abstract
 PDF
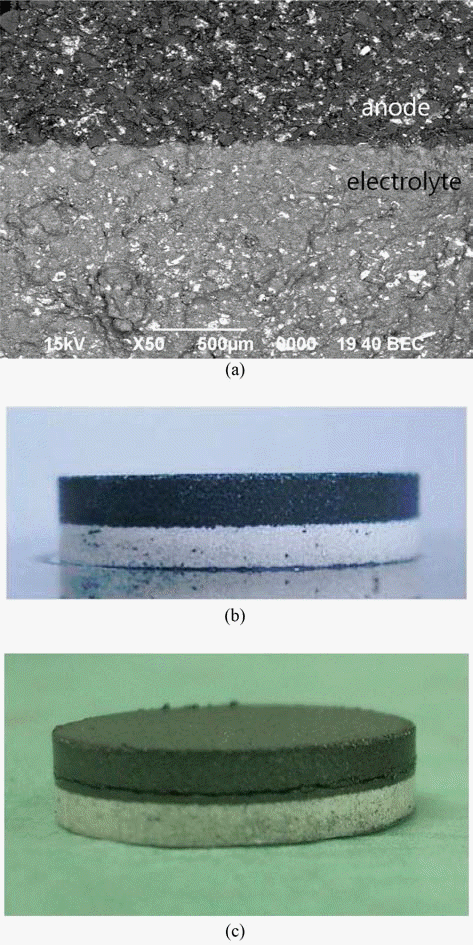
PDF The effects of particle size of Li-Si alloy and LiCl-KCl addition as a binder phase for raw material of anode were investigated on the formability of the thermal battery anode. The formability was evaluated with respect to filling density, tap density, compaction density, spring-back and compressive strength. With increasing particle size of Li-Si alloy powder, densities increased while spring-back and compressive strength decreased. Since the small spring-back is beneficial to avoiding breakage of pressed compacts, larger particles might be more suitable for anode forming. The increasing amount of LiCl-KCl binder phase contributed to reducing spring-back, improving the formability of anode powder too. The control of particle size also seems to be helpful to get double pressed pellets, which consisted of two layer of anode and electrolyte.
TOP
 KPMI
KPMI


 First
First Prev
Prev


