Search
- Page Path
- HOME > Search
- [Korean]
- Hydrogen Reduction Behavior of NCM-based Lithium-ion Battery Cathode Materials
- So-Yeong Lee, So-Yeon Lee, Dae-Hyeon Lee, Ho-Sang Sohn
- J Powder Mater. 2024;31(2):163-168. Published online April 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00017
- 1,561 View
- 41 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF - As the demand for lithium-ion batteries for electric vehicles is increasing, it is important to recover valuable metals from waste lithium-ion batteries. In this study, the effects of gas flow rate and hydrogen partial pressure on hydrogen reduction of NCM-based lithium-ion battery cathode materials were investigated. As the gas flow rate and hydrogen partial pressure increased, the weight loss rate increased significantly from the beginning of the reaction due to the reduction of NiO and CoO by hydrogen. At 700 °C and hydrogen partial pressure above 0.5 atm, Ni and Li2O were produced by hydrogen reduction. From the reduction product and Li recovery rate, the hydrogen reduction of NCM-based cathode materials was significantly affected by hydrogen partial pressure. The Li compounds recovered from the solution after water leaching of the reduction products were LiOH, LiOH·H2O, and Li2CO3, with about 0.02 wt% Al as an impurity.
-
Citations
Citations to this article as recorded by- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef
- Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
- [Korean]
- Synthesis and Investigation of LiVPO4O1-xFxvia Control of the Fluorine Content for Cathode of Lithium-ion Batteries
- Minkyung Kim, Dong-hee Lee, Changyu Yeo, Sooyeon Choi, Chiwon Choi, Hyunmin Yoon
- J Powder Mater. 2023;30(6):516-520. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.516
- 995 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF Highly safe lithium-ion batteries (LIBs) are required for large-scale applications such as electrical vehicles and energy storage systems. A highly stable cathode is essential for the development of safe LIBs. LiFePO4 is one of the most stable cathodes because of its stable structure and strong bonding between P and O. However, it has a lower energy density than lithium transition metal oxides. To investigate the high energy density of phosphate materials, vanadium phosphates were investigated. Vanadium enables multiple redox reactions as well as high redox potentials. LiVPO4O has two redox reactions (V5+/V4+/V3+) but low electrochemical activity. In this study, LiVPO4O is doped with fluorine to improve its electrochemical activity and increase its operational redox potential. With increasing fluorine content in LiVPO4O1-xFx, the local vanadium structure changed as the vanadium oxidation state changed. In addition, the operating potential increased with increasing fluorine content. Thus, it was confirmed that fluorine doping leads to a strong inductive effect and high operating voltage, which helps improve the energy density of the cathode materials.
- [Korean]
- Research Trends of Cathode Materials for Lithium-Ion Batteries used in Electric Vehicles
- Dong-Yo Shin, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2019;26(1):58-69. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.58
- 987 View
- 7 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
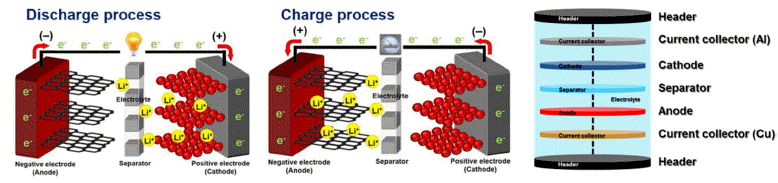
PDF High performance lithium-ion batteries (LIBs) have attracted considerable attention as essential energy sources for high-technology electrical devices such as electrical vehicles, unmanned drones, uninterruptible power supply, and artificial intelligence robots because of their high energy density (150-250 Wh/kg), long lifetime (> 500 cycles), low toxicity, and low memory effects. Of the high-performance LIB components, cathode materials have a significant effect on the capacity, lifetime, energy density, power density, and operating conditions of high-performance LIBs. This is because cathode materials have limitations with respect to a lower specific capacity and cycling stability as compared to anode materials. In addition, cathode materials present difficulties when used with LIBs in electric vehicles because of their poor rate performance. Therefore, this study summarizes the structural and electrochemical properties of cathode materials for LIBs used in electric vehicles. In addition, we consider unique strategies to improve their structural and electrochemical properties.
-
Citations
Citations to this article as recorded by- Estimation of Representative Mechanical Property of Porous Electrode for Secondary Batteries with Homogenization Method
Changmin Pyo, Jaewoong Kim
Journal of the Korean Society of Manufacturing Process Engineers.2022; 21(9): 85. CrossRef
- Estimation of Representative Mechanical Property of Porous Electrode for Secondary Batteries with Homogenization Method
- [Korean]
- Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
- Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2019;26(1):49-57. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.49
- 1,930 View
- 20 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
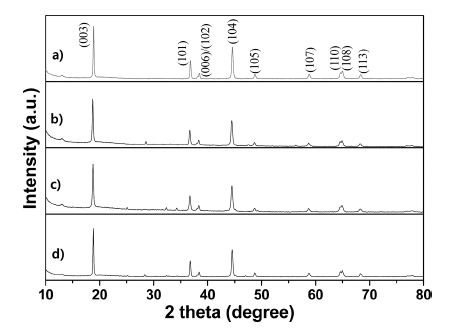
PDF Layered LiNi0.83Co0.11Mn0.06O2 cathode materials single- and dual-doped by the rare-earth elements Ce and Nd are successfully fabricated by using a coprecipitation-assisted solid-phase method. For comparison purposes, nondoping pristine LiNi0.83Co0.11Mn0.06O2 cathode material is also prepared using the same method. The crystal structure, morphology, and electrochemical performances are characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometer (EDS) mapping, and electrochemical techniques. The XRD data demonstrates that all prepared samples maintain a typical α-NaFeO2-layered structure with the
R-3m -
Citations
Citations to this article as recorded by- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
Heewon Choi, Nam-gyu Lim, Seong Jun Lee, Jungsoo Park
Journal of Mechanical Science and Technology.2021; 35(6): 2697. CrossRef - Synthesis of CeVO4-V2O5 nanowires by cation-exchange method for high-performance lithium-ion battery electrode
Xueliu Xu, Shiying Chang, Taofang Zeng, Yidan Luo, Dong Fang, Ming Xie, Jianhong Yi
Journal of Alloys and Compounds.2021; 887: 161237. CrossRef
- Numerical approach for lithium-ion battery performance considering various cathode active material composition for electric vehicles using 1D simulation
- [English]
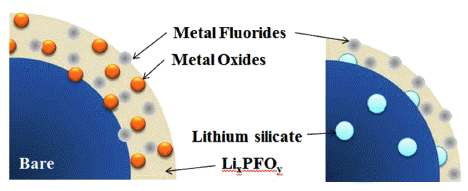
- Lithium-silicate coating on Lithium Nickel Manganese Oxide (LiNi0.7Mn0.3O2) with a Layered Structure
- Dong-jin Kim, Da-ye Yoon, Woo-byoung Kim, Jae-won Lee
- J Korean Powder Metall Inst. 2017;24(2):87-95. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.87
- 1,241 View
- 6 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Lithium silicate, a lithium-ion conducting ceramic, is coated on a layer-structured lithium nickel manganese oxide (LiNi0.7Mn0.3O2). Residual lithium compounds (Li2CO3 and LiOH) on the surface of the cathode material and SiO2 derived from tetraethylorthosilicate are used as lithium and silicon sources, respectively. Powder X-ray diffraction and scanning electron microscopy with energy-dispersive spectroscopy analyses show that lithium silicate is coated uniformly on the cathode particles. Charge and discharge tests of the samples show that the coating can enhance the rate capability and cycle life performance. The improvements are attributed to the reduced interfacial resistance originating from suppression of solid-electrolyte interface (SEI) formation and dissolution of Ni and Mn due to the coating. An X-ray photoelectron spectroscopy study of the cycled electrodes shows that nickel oxide and manganese oxide particles are formed on the surface of the electrode and that greater decomposition of the electrolyte occurs for the bare sample, which confirms the assumption that SEI formation and Ni and Mn dissolution can be reduced using the coating process.
-
Citations
Citations to this article as recorded by- Artificial cathode-electrolyte interphases on nickel-rich cathode materials modified by silyl functional group
Hye Ji Song, Seol Heui Jang, Juhyeon Ahn, Si Hyoung Oh, Taeeun Yim
Journal of Power Sources.2019; 416: 1. CrossRef
- Artificial cathode-electrolyte interphases on nickel-rich cathode materials modified by silyl functional group
- [Korean]
- Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
- Woonghee Choi, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(4):287-296. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.287
- 1,813 View
- 33 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Ni1/3Co1/3Mn1/3(OH)2 powders have been synthesized in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH using NH4OH as a chelating agent. The co-precipitation temperature is varied in the range of 30-80°C. Calcination of the prepared precursors with Li2CO3 for 8 h at 1000°C in air results in Li Ni1/3Co1/3Mn1/3O2 powders. Two kinds of obtained powders have been characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analyzer, and tap density measurements. The co-precipitation temperature does not differentiate the XRD patterns of precursors as well as their final powders. Precursor powders are spherical and dense, consisting of numerous acicular or flaky primary particles. The precursors obtained at 70 and 80°C possess bigger primary particles having more irregular shapes than those at lower temperatures. This is related to the lower tap density measured for the former. The final powders show a similar tendency in terms of primary particle shape and tap density. Electrochemical characterization shows that the initial charge/discharge capacities and cycle life of final powders from the precursors obtained at 70 and 80°C are inferior to those at 50°C. It is concluded that the optimum co-precipitation temperature is around 50°C.
-
Citations
Citations to this article as recorded by- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
Seon Hwa Lee, Ki Young Kwon, Byeong Kil Choi, Hyun Deog Yoo
Journal of Electroanalytical Chemistry.2022; 924: 116828. CrossRef
- A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries
- [Korean]
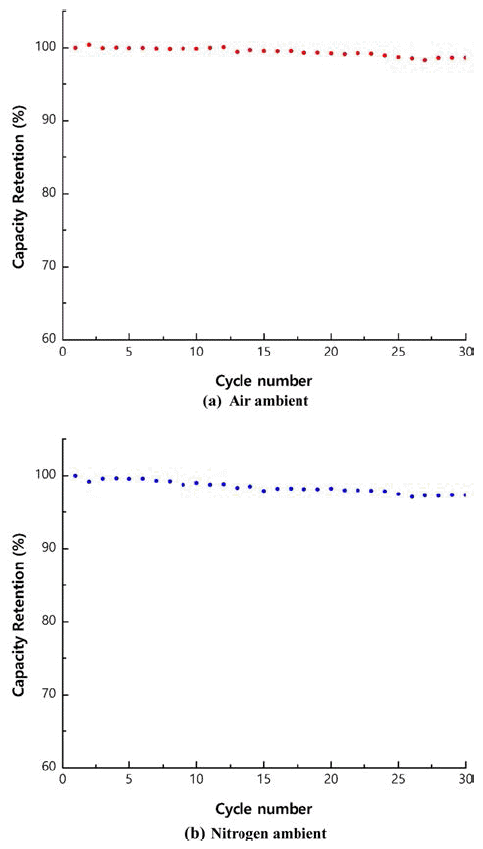
- Characteristics of Ni1/3Co1/3Mn1/3(OH)2 Powders Prepared by Co-Precipitation in Air and Nitrogen Atmospheres
- Woonghee Choi, Se-Ryen Park, Chan Hyoung Kang
- J Korean Powder Metall Inst. 2016;23(2):136-142. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.136
- 2,649 View
- 53 Download
- 6 Citations
-
 Abstract
Abstract
 PDF
PDF As precursors of cathode materials for lithium ion batteries, Ni1/3Co1/3Mn1/3(OH)2 powders are prepared in a continuously stirred tank reactor via a co-precipitation reaction between aqueous metal sulfates and NaOH in the presence of NH4OH in air or nitrogen ambient. Calcination of the precursors with Li2CO3 for 8 h at 1,000°C in air produces dense spherical cathode materials. The precursors and final powders are characterized by X-ray diffraction (XRD), scanning electron microscopy, particle size analysis, tap density measurement, and thermal gravimetric analysis. The precursor powders obtained in air or nitrogen ambient show XRD patterns identified as Ni1/3Co1/3Mn1/3(OH)2. Regardless of the atmosphere, the final powders exhibit the XRD patterns of LiNi1/3Co1/3Mn1/3O2 (NCM). The precursor powders obtained in air have larger particle size and lower tap density than those obtained in nitrogen ambient. NCM powders show similar tendencies in terms of particle size and tap density. Electrochemical characterization is performed after fabricating a coin cell using NCM as the cathode and Li metal as the anode. The NCM powders from the precursors obtained in air and those from the precursors obtained in nitrogen have similar initial charge/discharge capacities and cycle life. In conclusion, the powders co-precipitated in air can be utilized as precursor materials, replacing those synthesized in the presence of nitrogen injection, which is the usual industrial practice.
-
Citations
Citations to this article as recorded by- Thermodynamic modeling and parameters optimization for Ni0.8Co0.1Mn 0.1(OH)2 synthesis via transitional metal coprecipitation
Zanlang Tang, Chen Liu, Xincun Tang, Haonan Liu, Biao Qin
Materials Science and Engineering: B.2026; 326: 119205. CrossRef - Stabilization of High Nickel Cathode Materials with Core-Shell Structure via Co-precipitation Method
Minjeong Kim, Soonhyun Hong, Heongkwon Jeon, Jahun Koo, Heesang Lee, Gyuseok Choi, Chunjoong Kim
Korean Journal of Materials Research.2022; 32(4): 216. CrossRef - Spherical agglomeration of nickel-manganese-cobalt hydroxide in turbulent Batchelor vortex flow
Xiaotong Sun, Jinsoo Kim, Woo-Sik Kim
Chemical Engineering Journal.2021; 421: 129924. CrossRef - Design strategies for development of nickel-rich ternary lithium-ion battery
Kyu Hwan Choi, Xuyan Liu, Xiaohong Ding, Qiang Li
Ionics.2020; 26(3): 1063. CrossRef - Effect of Single and Dual Doping of Rare Earth Metal Ce and Nd Elements on Electrochemical Properties of LiNi0.83 Co0.11Mn0.06O2 Cathode Lithium-ion Battery Material
Yoo-Young Kim, Jong-Keun Ha, Kwon-Koo Cho
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 49. CrossRef - Effects of Precursor Co-Precipitation Temperature on the Properties of LiNi1/3Co1/3Mn1/3O2 Powders
Woonghee Choi, Chan Hyoung Kang
Journal of Korean Powder Metallurgy Institute.2016; 23(4): 287. CrossRef
- Thermodynamic modeling and parameters optimization for Ni0.8Co0.1Mn 0.1(OH)2 synthesis via transitional metal coprecipitation
- [Korean]
- Fabrication of LiNiO2 using NiSO4 Recovered from NCM (Li[Ni,Co,Mn]O2) Secondary Battery Scraps and Its Electrochemical Properties
- Yong-Gyu Kwag, Mi-So Kim, Yoo-Young Kim, Im-Sic Choi, Dong-Kyu Park, In-Sup Ahn, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2014;21(4):286-293. Published online August 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.4.286

- 859 View
- 3 Download
-
 Abstract
Abstract
 PDF
PDF The electrochemical properties of cells assembled with the LiNiO2 (LNO) recycled from cathode materials of waste lithium secondary batteries (Li[Ni,Co,Mn]O2), were evaluated in this study. The leaching, neutralization and solvent extraction process were applied to produce high-purity NiSO4 solution from waste lithium secondary batteries. High-purity NiO powder was then fabricated by the heat-treatment and mixing of the NiSO4 solution and H2C2O4. Finally, LiNiO2 as a cathode material for lithium ion secondary batteries was synthesized by heat treatment and mixing of the NiO and Li2CO3 powders. We assembled the cells using the LiNiO2 powders and evaluated the electrochemical properties. Subsequently, we evaluated the recycling possibility of the cathode materials for waste lithium secondary battery using the processes applied in this work.
- [Korean]
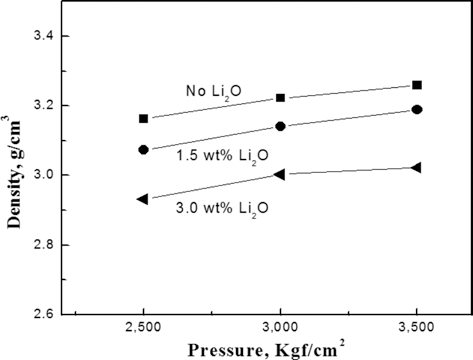
- Effects of Li2O Addition and Heat-Treatment on Formability of FeS2 Powder for Cathode of Thermal Battery
- Sung-Soo Ryu, Won-Jin Lee, Seongwon Kim, Hae-Won Cheong, Sung-Baek Cho, Seung-Ho Kang, Sung-Min Lee
- J Korean Powder Metall Inst. 2014;21(3):185-190. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.185
- 683 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF FeS2 has been widely used for cathode materials in thermal battery because of its high stability and current capability at high operation temperature. Salts such as a LiCl-KCl were added as a binder for improving electrical performance and formability of FeS2 cathode powder. In this study, the effects of the addition of Li2O in LiCl-KCl binder on the formability of FeS2 powder compact were investigated. With the increasing amount of Li2O addition to LiCl-KCl binder salts, the strength of the pressed compacts increased considerably when the powder mixture were pre-heat-treated above 350°C. The heat-treatment resulted in promoting the coating coverage of FeS2 particles by the salts as Li2O was added. The observed coating as Li2O addition might be attributed to the enhanced wettability of the salt rather than its reduced melting temperature. The high strength of compacts by the Li2O addition and pre-heat-treatment could improve the formability of FeS2 raw materials.
-
Citations
Citations to this article as recorded by- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
Sung-Soo Ryu, Hui-Sik Kim, Seongwon Kim, Hyung-Tae Kim, Hae-Won Cheong, Sung-Min Lee
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 331. CrossRef
- Effects of Particle Size and Binder Phase Addition on Formability of Li-Si Alloy Powder for Thermal Battery Anode
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
- Jae-won Lee, Dae Weon Kim, Seong Tae Jang
- J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
- 580 View
- 3 Download
-
 Abstract
Abstract
 PDF
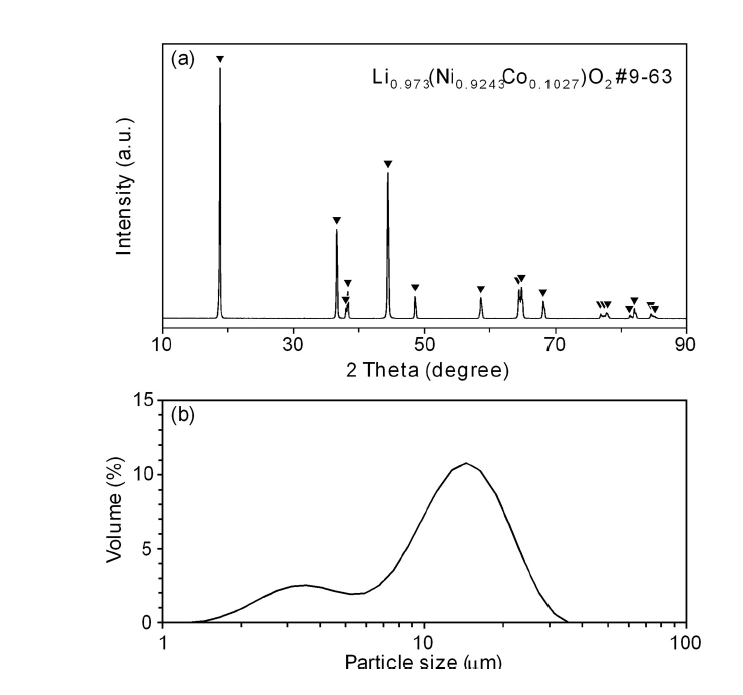
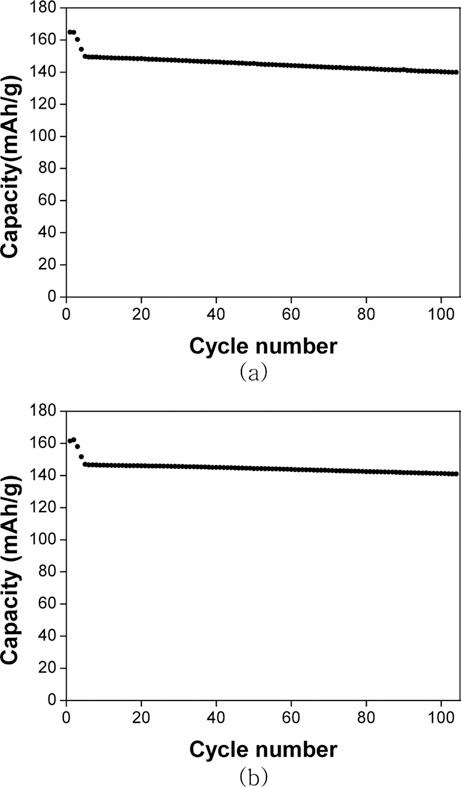
PDF Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
TOP
 KPMI
KPMI


 First
First Prev
Prev


