Search
- Page Path
- HOME > Search
- [Korean]
- Microstructure and Characterization of Overlay Welding Layer using Fe-based Composite Powders
- Hong Min, Jong-Jae Lee, Jin Kyu Lee
- J Korean Powder Metall Inst. 2019;26(3):214-219. Published online June 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.3.214
- 748 View
- 3 Download
-
 Abstract
Abstract
 PDF
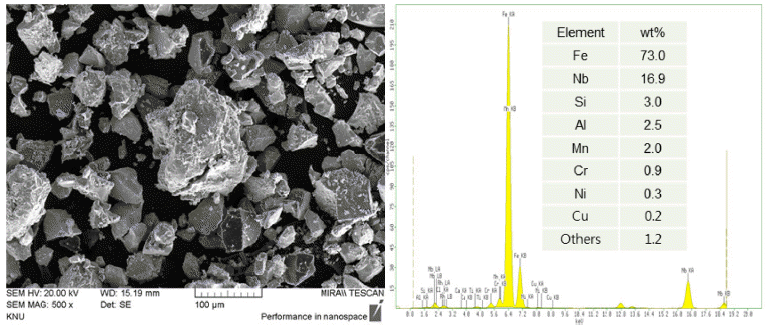
PDF In this study, the microstructure and characterization of an overlay welding layer using Fe-based composite powders are reported. The effects of the number of passes and composition of powders on the microstructure and mechanical properties are investigated in detail. The welding wire and powders are deposited twice on a stainless-steel rod using a laser overlay welding process. The microstructure and structural characterization are performed by scanning electron microscopy and X-ray diffraction. The mechanical properties of the first and second overlay layers are analyzed through the micro-Vickers-hardness tester and abrasion wear tester. In the second overlay layer, the hardness and specific wear are approximately 840 Hv and 2.0 × 10−5 mm3/Nm, respectively. It is suggested that the increase of the volume fractions of (Cr,Fe)7C3 and NbC phases in the second welding layer enhances the hardness and wear resistance.
- [Korean]
- Development of Metal Composite Powder Non-corrosive Flux for Low Temperature Forming of the Aluminum Brazing Filler Material
- Dae-Young Kim, Ha-Neul Jang, Dae-Ho Yoon, Yun-Ho Shin, Seong-Ho Kim, Hyun-Joo Choi
- J Korean Powder Metall Inst. 2019;26(1):16-21. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.16
- 788 View
- 8 Download
-
 Abstract
Abstract
 PDF
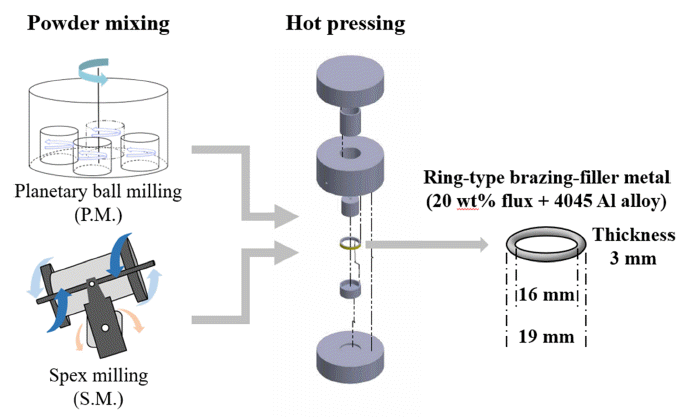
PDF In aluminum brazing processes, corrosive flux, which is used in preventing oxidation, is currently raising environmental concerns because it generates many pollutants such as dioxin. The brazing process involving noncorrosive flux is known to encounter difficulties because the melting temperature of the flux is similar to that of the base material. In this study, a new brazing filler material is developed based on aluminum and non-corrosive flux composite powder. To minimize the interference of consolidation aluminum alloy powder by the flux, the flux is intentionally embedded in the aluminum alloy powder using a mechanical milling process. This study demonstrates that the morphology of the composite powder can be varied according to the mixing process, and this significantly affects the relative density and mechanical properties of the final filler samples.
- [Korean]
- Fabrication of Mo-Cu Powders by Ball Milling and Hydrogen Reduction of MoO3-CuO Powder Mixtures
- Hyunji Kang, Sung-Tag Oh
- J Korean Powder Metall Inst. 2018;25(4):322-326. Published online August 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.4.322
- 913 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
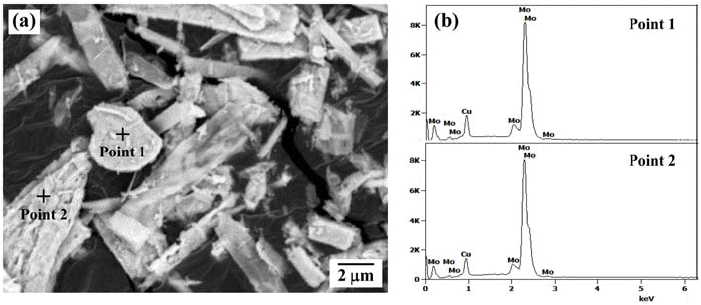
PDF The hydrogen reduction behavior of MoO3-CuO powder mixture for the synthesis of homogeneous Mo-20 wt% Cu composite powder is investigated. The reduction behavior of ball-milled powder mixture is analyzed by XRD and temperature programmed reduction method at various heating rates in Ar-10% H2 atmosphere. The XRD analysis of the heat-treated powder at 300°C shows Cu, MoO3, and Cu2MoO5 phases. In contrast, the powder mixture heated at 400°C is composed of Cu and MoO2 phases. The hydrogen reduction kinetic is evaluated by the amount of peak shift with heating rates. The activation energies for the reduction, estimated by the slope of the Kissinger plot, are measured as 112.2 kJ/mol and 65.2 kJ/mol, depending on the reduction steps from CuO to Cu and from MoO3 to MoO2, respectively. The measured activation energy for the reduction of MoO3 is explained by the effect of pre-reduced Cu particles. The powder mixture, hydrogen-reduced at 700°C, shows the dispersion of nano-sized Cu agglomerates on the surface of Mo powders.
-
Citations
Citations to this article as recorded by- Synthesis of Mo-Cu nanocomposite powder by hydrogen reduction of copper nitrate coated MoO3 powder mixture
Ji Won Choi, Ji Young Kim, Youngmin Kim, Eui Seon Lee, Sung-Tag Oh
Materials Letters.2024; 377: 137565. CrossRef - Fabrication of Porous Mo-Cu by Freeze Drying and Hydrogen Reduction of Metal Oxide Powders
Hyunji Kang, Ju-Yeon Han, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 1. CrossRef - Hydrogen Reduction Behavior and Microstructure Characteristics of Ball-milled CuO-Co3O4 Powder Mixtures
Ju-Yeon Han, Gyuhwi Lee, Hyunji Kang, Sung-Tag Oh
Journal of Korean Powder Metallurgy Institute.2019; 26(5): 410. CrossRef
- Synthesis of Mo-Cu nanocomposite powder by hydrogen reduction of copper nitrate coated MoO3 powder mixture
- [Korean]
- Characteristics of WO3-CuO Powder Mixture Prepared by High-Energy Ball Milling in a Bead Mill for the Synthesis of W-Cu Nanocomposite Powder
- Hae-Ryong Park, Sung-Soo Ryu
- J Korean Powder Metall Inst. 2017;24(5):406-413. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.406
- 1,032 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
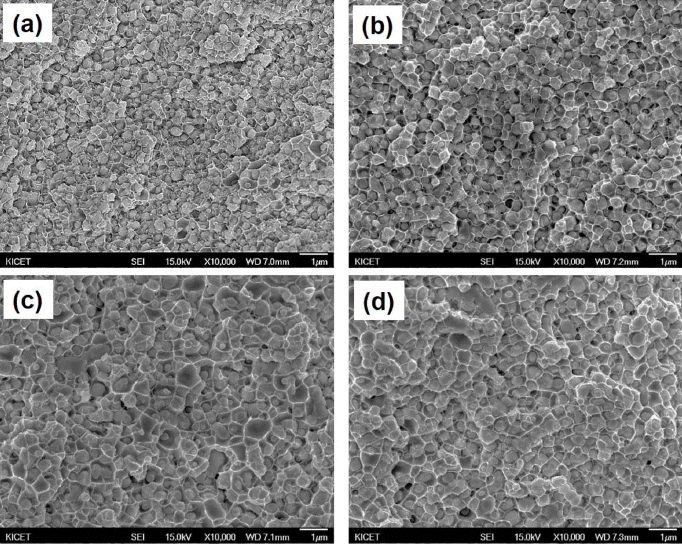
PDF A Nanosized WO3 and CuO powder mixture is prepared using novel high-energy ball milling in a bead mill to obtain a W-Cu nanocomposite powder, and the effect of milling time on the structural characteristics of WO3-CuO powder mixtures is investigated. The results show that the ball-milled WO3-CuO powder mixture reaches at steady state after 10 h milling, characterized by the uniform and narrow particle size distribution with primary crystalline sizes below 50 nm, a specific surface area of 37 m2/g, and powder mean particle size (D50) of 0.57 μm. The WO3-CuO powder mixtures milled for 10 h are heat-treated at different temperatures in H2 atmosphere to produce W-Cu powder. The XRD results shows that both the WO3 and CuO phases can be reduced to W and Cu phases at temperatures over 700°C. The reduced W-Cu nanocomposite powder exhibits excellent sinterability, and the ultrafine W-Cu composite can be obtained by the Cu liquid phase sintering process.
-
Citations
Citations to this article as recorded by- Morphological Characteristics of W/Cu Composite Nanoparticles with Complex Phase Structure Synthesized via Reactive Radio Frequency (RF) Thermal Plasma
Chulwoong Han, Song-Yi Kim, Soobin Kim, Ji-Woon Lee
Metals.2024; 14(9): 1070. CrossRef
- Morphological Characteristics of W/Cu Composite Nanoparticles with Complex Phase Structure Synthesized via Reactive Radio Frequency (RF) Thermal Plasma
- [Korean]
- Fabrication of Fe3O4/Fe/Graphene nanocomposite powder by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ji-Seub Choi, Hoi-Jin Lee, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2017;24(4):308-314. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.308
- 1,010 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
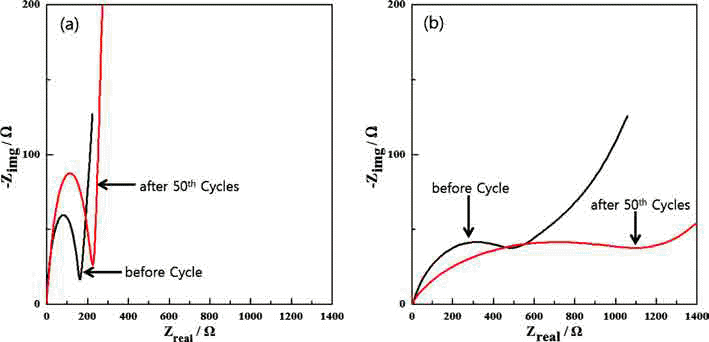
PDF Fe3O4/Fe/graphene nanocomposite powder is synthesized by electrical wire explosion of Fe wire and dispersed graphene in deionized water at room temperature. The structural and electrochemical characteristics of the powder are characterized by the field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, field-emission transmission electron microscopy, cyclic voltammetry, and galvanometric discharge-charge method. For comparison, Fe3O4/Fe nanocomposites are fabricated under the same conditions. The Fe3O4/Fe nanocomposite particles, around 15-30 nm in size, are highly encapsulated in a graphene matrix. The Fe3O4/Fe/graphene nanocomposite powder exhibits a high initial charge specific capacity of 878 mA/g and a high capacity retention of 91% (798 mA/g) after 50 cycles. The good electrochemical performance of the Fe3O4/Fe/graphene nanocomposite powder is clearly established by comparison of the results with those obtained for Fe3O4/Fe nanocomposite powder and is attributed to alleviation of volume change, good distribution of electrode active materials, and improved electrical conductivity upon the addition of graphene.
-
Citations
Citations to this article as recorded by- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
Kyunbae Lee, Joonsik Lee, Byung Mun Jung, Byeongjin Park, Taehoon Kim, Sang Bok Lee
Applied Surface Science.2019; 478: 733. CrossRef
- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
- [Korean]
- Sintering of Fe-30 wt% TiC Composite Powders Fabricated from (Fe, TiH2, C) Powder Mixture
- Byunghoon Lee, Ji Soon Kim
- J Korean Powder Metall Inst. 2015;22(5):356-361. Published online October 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.5.356
- 883 View
- 2 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
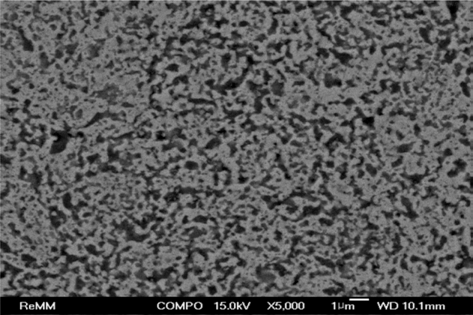
PDF Fe-30 wt% TiC composite powders are fabricated by in situ reaction synthesis after planetary ball milling of (Fe, TiH2, Carbon) powder mixture. Two sintering methods of a pressureless sintering and a spark-plasma sintering are tested to densify the Fe-30 wt% TiC composite powder compacts. Pressureless sintering is performed at 1100, 1200 and 1300°C for 1-3 hours in a tube furnace under flowing argon gas atmosphere. Spark-plasma sintering is carried out under the following condition: sintering temperature of 1050°C, soaking time of 10 min, sintering pressure of 50 MPa, heating rate of 50°C/min, and in a vacuum of 0.1 Pa. The curves of shrinkage and its derivative (shrinkage rate) are obtained from the data stored automatically during sintering process. The densification behaviors are investigated from the observation of fracture surface and cross-section of the sintered compacts. The pressureless-sintered powder compacts are not densified even after sintering at 1300°C for 3 h, which shows a relative denstiy of 66.9%. Spark-plasma sintering at 1050°C for 10 min exhibits nearly full densification of 99.6% relative density under the sintering pressure of 50 MPa.
-
Citations
Citations to this article as recorded by- Abrasive Wear Performance of Spherical Hierarchical Structured TiC/High-Manganese Steel Composites
Tao He, Shengnian Zhao, Dehong Lu, Yehua Jiang, Mojin Zhou
Materials.2024; 18(1): 130. CrossRef - Effect of TiC particle size on high temperature oxidation behavior of TiC reinforced stainless steel
Yeong-Hwan Lee, Sungmin Ko, Hyeonjae Park, Donghyun Lee, Sangmin Shin, Ilguk Jo, Sang-Bok Lee, Sang-Kwan Lee, Yangdo Kim, Seungchan Cho
Applied Surface Science.2019; 480: 951. CrossRef - Effect of TiC addition on surface oxidation behavior of SKD11 tool steel composites
Seungchan Cho, Ilguk Jo, Heebong Kim, Hyuk-Tae Kwon, Sang-Kwan Lee, Sang-Bok Lee
Applied Surface Science.2017; 415: 155. CrossRef
- Abrasive Wear Performance of Spherical Hierarchical Structured TiC/High-Manganese Steel Composites
- [Korean]
- Manufacturing and Properties of CGI-based Composite Coating Layer Utilizing a Warm Spray Process and Cu-Ga and Cu-In Mixed Powders
- Min-Gwang Jeon, Myeong-Ju Lee, Hyeong-Jun Kim, Kee-Ahn Lee
- J Korean Powder Metall Inst. 2014;21(3):229-234. Published online June 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.3.229
- 819 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
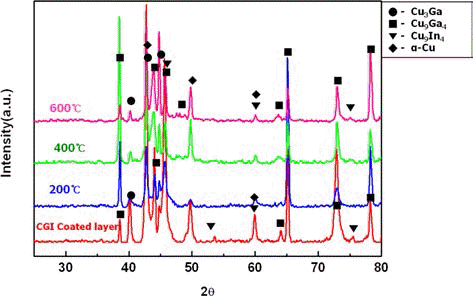
PDF This study manufactured a CIG-based composite coating layer utilizing a new warm spray process, and a mixed powder of Cu-20at.%Ga and Cu-20at.%In. In order to obtain the mixed powder with desired composition, the Cu-20at.%Ga and Cu-20at.%In powders were mixed with a 7:1 ratio. The mixed powder had an average particle size of 35.4 μm. Through the utilization of a warm spray process, a CIG-based composite coating layer of 180 μm thickness could be manufactured on a pure Al matrix. To analyze the microstructure and phase, the warm sprayed coating layer underwent XRD, SEM/EDS and EMPA analyses. In addition, to improve the physical properties of the coating layer, an annealing heat treatment was conducted at temperatures of 200°C, 400°C and 600°C for 1 hour each. The microstructure analysis identified α-Cu, Cu4In and Cu3Ga phases in the early mixed powder, while Cu4In disappeared, and additional Cu9In4 and Cu9Ga4 phases were identified in the warm sprayed coating layer. Porosity after annealing heat treatment reduced from 0.75% (warm sprayed coating layer) to 0.6% (after 600°C/1 hr. heat treatment), and hardness reduced from 288 Hv to 190 Hv. No significant phase changes were found after annealing heat treatment.
-
Citations
Citations to this article as recorded by- Fabrication and Microstructure/Properties of Bulk-typeTantalum Material by a Kinetic Spray Process
Ji-Hye Lee, Ji-Won Kim, Kee-Ahn Lee
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 8. CrossRef - Effect of Heat Treatment Environment on the Microstructure and Properties of Kinetic Sprayed Tantalum Coating Layer
Ji-Hye Lee, Hyung-Jun Kim, Kee-Ahn Lee
Journal of Korean Powder Metallurgy Institute.2015; 22(1): 32. CrossRef
- Fabrication and Microstructure/Properties of Bulk-typeTantalum Material by a Kinetic Spray Process
- [English]
- Coating of Cobalt Over Tungsten Carbide Powder by Wet Chemical Reduction Method
- Hyun-Seon Hong, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2014;21(2):93-96. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.93
- 1,644 View
- 8 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF Cobalt coated tungsten carbide-cobalt composite powder has been prepared through wet chemical reduction method. The cobalt sulfate solution was converted to the cobalt chloride then the cobalt hydroxide. The tungsten carbide powders were added in to the cobalt hydroxide, the cobalt hydroxide was reduced and coated over tungsten carbide powder using hypo-phosphorous acid. Both the cobalt and the tungsten carbide phase peaks were evident in the tungsten carbide-cobalt composite powder by X-ray diffraction. The average particle size measured via scanning electron microscope, particle size analysis was around 380 nm and the thickness of coated cobalt was determined to be 30~40 nm by transmission electron microscopy.
-
Citations
Citations to this article as recorded by- Electroless Ni-P deposition on WC powders through direct PdCl2 activation and study on the underlying mechanisms
Peng Tang, Shuwen Jiang, Jiawei Yan, Xianquan Li
Next Materials.2025; 6: 100496. CrossRef - Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef - Spark plasma sintering of WC–Co tool materials prepared with emphasis on WC core–Co shell structure development
Sungkyu Lee, Hyun Seon Hong, Hyo-Seob Kim, Soon-Jik Hong, Jin-Ho Yoon
International Journal of Refractory Metals and Hard Materials.2015; 53: 41. CrossRef
- Electroless Ni-P deposition on WC powders through direct PdCl2 activation and study on the underlying mechanisms
- [Korean]
- Synthesis of Pt@TiO2 Nano-composite via Photochemical Reduction Method
- Ji Young Kim, Jong Min Byun, Jin Woo Kim, Young Do Kim
- J Korean Powder Metall Inst. 2014;21(2):119-123. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.119
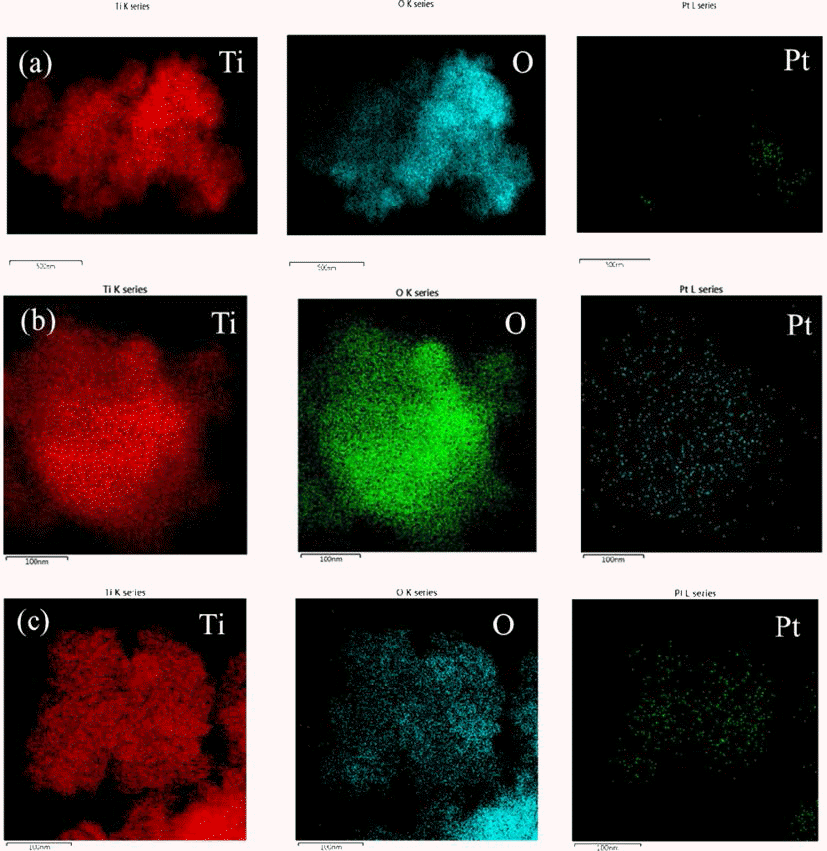
- 826 View
- 3 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Pt has been widely used as catalyst for fuel cell and exhausted gas clean systems due to its high catalytic activity. Recently, there have been researches on fabricating composite materials of Pt as a method of reducing the amount of Pt due to its high price. One of the approaches for saving Pt used as catalyst is a core shell structure consisting of Pt layer on the core of the non-noble metal. In this study, the synthesis of Pt shell was conducted on the surface of TiO2 particle, a non-noble material, by applying ultraviolet (UV) irradiation. Anatase TiO2 particles with the average size of 20~30 nm were immersed in the ethanol dissolved with Pt precursor of H2PtCl6∙6H2O and exposed to UV irradiation with the wavelength of 365 nm. It was confirmed that Pt nano-particles were formed on the surface of TiO2 particles by photochemical reduction of Pt ion from the solution. The morphology of the synthesized Pt@TiO2 nano-composite was examined by TEM (Transmission Electron Microscopy).
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef - Synthesis and Photo Catalytic Activity of 10 wt%, 20 wt%Li-TiO2 Composite Powders
Hyeong-Chul Kim, Jae-Kil Han
Journal of Korean Powder Metallurgy Institute.2016; 23(1): 33. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


